Chapter 14: Introduction to Community Ecology
- Page ID
- 92866
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)-
Develop an appreciation of the interconnected nature of ecological communities
-
Organize species interactions based on their impacts on the species involved
-
Understand some of the factors that structure ecological communities
Introduction to Species Interactions
Populations rarely, if ever, live in isolation from populations of other species. All populations occupying the same habitat form a community (populations of multiple species inhabiting a specific area at the same time). The number of species occupying the same habitat and their relative abundance is known as species diversity. Areas with low diversity, such as the glaciers of Antarctica, still contain a wide variety of living things, whereas the diversity of tropical rainforests is so great that it cannot be counted. Ecology is studied at the community level to understand how species interact with each other and compete for the same resources.
The interactions among populations of different species play a major role in regulating population growth and abundance. A species interaction is the effect that a pair of organisms living together in a community have on each other. Interactions range from mutualism, which benefits both species involved, to competition, which harms both species involved (Wootton and Emmerson 2005). Interactions can be indirect, through intermediaries such as shared resources or common enemies. All of these interactions can be organized by the effects the species have on each other (Figure 1).
Species interactions may be short-term, like pollination and predation, or long-term; both often strongly influence the evolution of the species involved. Short-term interactions are short-lived in terms of the duration of a single interaction: a predator kills and eats a prey; a pollinator transfers pollen from one flower to another; but they are extremely durable in terms of their influence on the evolution of both partners. As a result, the partners coevolve (Bengtson 2002, Lunau 2004).

Antagonistic Interactions
Predation
In predation, one organism, the predator, kills and eats another organism, its prey. Predators are adapted and often highly specialized for hunting, with acute senses such as vision, hearing, or smell. Many predatory animals, both vertebrate and invertebrate, have sharp claws or jaws to grip, kill, and cut up their prey. Other adaptations include stealth and aggressive mimicry that improve hunting efficiency. Predation has a powerful selective effect on prey, causing them to develop antipredator adaptations such as warning coloration, alarm calls and other signals, camouflage and defensive spines and chemicals (Royal Saskatchewan Museum 2012, Bar-Yam 2018, Vermeij 1993). Predation has been a major driver of evolution since at least the Cambrian period (Bengston 2002). Predators control the population dynamics of their prey and vice versa and ecologists model these dynamics using coupled equations known as the Lotka-Volterra predator-prey model. These models will be covered in more detail in the chapter on Antagonistic Interactions.



Parasitism
Parasitism is a relationship between species, where one organism, the parasite, lives on or in another organism, the host, causing it some harm, and is adapted structurally to this way of life (Poulin 2007). The parasite either feeds on the host, or, in the case of intestinal parasites, consumes some of its food (Martin and Schwab 2013). Not all parasites kill their hosts, but some do. Parasitoids are parasites that lay their eggs within a host. The larvae of parasitoids eventually hatch out of the host’s body, killing the host.
Competition
Competition can be defined as an interaction between organisms or species, in which the fitness of one is lowered by the presence of another. Competition is often for a resource such as food, water, or territory in limited supply, or for access to females for reproduction (Begon et al. 1996). Competition among members of the same species is known as intraspecific competition, while competition between individuals of different species is known as interspecific competition.
According to the competitive exclusion principle, no two species with the same ecological niche can coexist, and the species less suited to compete for resources should either adapt or die out (Hardin 1960; Pocheville 2015). Competition within and between species for resources plays a critical role in natural selection (Sahney et al. 2010). Ecologists model competition using the Lotka-Volterra competition model, and use this model to predict the conditions under which two species will coexist or one species outcompetes the other. These models will be covered in more detail in the chapter on Competition.

Beneficial Interactions
Mutualism
A mutualism is an interaction between two or more species, where both species derive a mutual benefit. One or both species involved in the interaction may be obligate, meaning they cannot survive in the short or long term without the other species (obligate mutualism). In other cases, though both species benefit, they may not need the mutualistic interaction to survive (facultative mutualism). Though mutualism has historically received less attention than other interactions such as predation (Begon et al. 1996), it is an ecologically important interaction.


Pollination and Seed Dispersal
In pollination, pollinators including insects (entomophily), some birds (ornithophily), and some bats, transfer pollen from a male flower part to a female flower part, enabling fertilization, in return for a reward of pollen or nectar (CropsReview.Com 2015). Plants and pollinators are often coevolved (Ehrlich and Raven 1964; Pollan 2001; Lunau 2004). Insect-pollinated flowers have bright colors, patterns, scent, nectar, and sticky pollen to attract insects, guide them to pick up and deposit pollen, and reward pollinators. Conversely, pollinator insects like bees are adapted to detect flowers by color, pattern, and scent, to collect pollen (such as with bristles shaped to form pollen baskets on their hind legs), and nectar.

Seed dispersal is the movement, spread or transport of seeds. Plants have limited mobility and rely upon a variety of dispersal vectors to transport their propagules, including both abiotic vectors such as the wind and living (biotic) vectors like birds (Lim ad Burns 2021) patterns of seed dispersal are determined in large part by the dispersal mechanism and this has important implications for the demographic and genetic structure of plant populations, as well as migration patterns and species interactions. There are five main modes of seed dispersal: gravity, wind, ballistic, water, and animals.
Other Types of Interactions
Amensalism
Amensalism (a term introduced by Haskell; Toepfer) is an interaction where an organism inflicts harm to another organism without any costs or benefits received by itself (Willey et al. 2013). Amensalism describes the adverse effect that one organism has on another organism. A classic example of amensalism is where sheep or cattle trample grass. Whilst the presence of the grass causes negligible detrimental effects to the animal's hoof, the grass suffers from being crushed.
Amensalism is often used to describe strongly asymmetrical competitive interactions, such as has been observed between the Spanish ibex and weevils of the genus Timarcha which feed upon the same type of shrub. Whilst the presence of the weevil has almost no influence on food availability, the presence of ibex has an enormous detrimental effect on weevil numbers, as they consume significant quantities of plant matter and incidentally ingest the weevils upon it (Gómez and González-Megías 2002).
Commensalism
Commensalism benefits one organism while the other organism neither benefits nor is harmed. It often occurs when one organism benefits from an interaction with another organism that is not affected. A good example is a remora living with a manatee. Remoras feed on the manatee's feces. The manatee is not affected by this interaction, as the remora does not deplete the manatee's resources (Williams and Williams 2003).

The southern masked-weaver bird is starting to make a nest in a tree in Zambezi Valley, Zambia. This is an example of a commensal relationship, in which one species (the bird) benefits, while the other (the tree) neither benefits nor is harmed. (credit: “Hanay”/Wikimedia Commons)

Figure 9: Foundational species increase food web complexity by facilitating species higher in the food chain. (A) Seven ecosystems with foundation species were sampled: coastal (seagrass, blue mussel, cordgrass), freshwater (watermilfoil, water-starwort) and terrestrial (Spanish moss, marram grass). (B) Food webs were constructed for both bare and foundation species-dominated replicate areas. (C) From each foundation species structured-food web, nodes (species) were randomly removed until the species number matched the species number of the bare food webs.
Although we often focus on trophic (food-related) interactions among species, there is growing evidence that non-trophic interactions can indirectly affect food web topology and trophic dynamics by affecting the species in the network and the strength of trophic links (Sanders et al. 2014; Kefi et al. 2015; van der Zee et al. 2016). Some examples of non-trophic interactions are habitat modification and competition for space.
Foundation species are spatially dominant habitat-structuring organisms (Dayton 1972; Governar 2010; Angelini et al. 2011). Although foundation species are part of the food web like any other species (e.g. as prey or predator), numerous studies have shown that they strongly facilitate the associated community by creating new habitat and alleviating physical stress (Bertness et al. 1999; Jones et al. 2010; Reid and Lortie 2012; Angelini and Silliman 2014; Kefi et al. 2015; van der Zee EM et al. 2015; van der Zee et al. 2016; Filazzola et al. 2017). This form of non-trophic facilitation by foundation species has been found to occur across a wide range of ecosystems and environmental conditions (Bertness and Callaway 1994; Bruno et al. 2003). In harsh coastal zones, corals, kelps, mussels, oysters, seagrasses, mangroves, and salt marsh plants facilitate organisms by attenuating currents and waves, providing aboveground structure for shelter and attachment, concentrating nutrients, and/or reducing desiccation stress during low tide exposure (Bertness and Callaway 1992; Angelini et al. 2011). In more benign systems, foundation species such as the trees in a forest, shrubs and grasses in savannahs, and macrophytes in freshwater systems, have also been found to play a major habitat-structuring role (Jeppesen et al. 1992; Bertness and Callaway 1994; Bruno et al. 2003; Ellison et al. 2005). Ultimately, all foundation species increase habitat complexity and availability, thereby partitioning and enhancing the niche space available to other species (Bruno et al. 2003; Bulleri et al. 2016; Borst et al. 2018).
Borst et al. (2018) tested the general hypothesis that foundation species modify food webs by enhancing their size as indicated by species number, and their complexity as indicated by link density, via facilitation of species, regardless of ecosystem type (Figure 6). Additionally, Borst et al. 2018 examined whether any change in food web properties caused by foundation species occurs via random facilitation of species throughout the entire food web or via targeted facilitation of specific species that belong to certain trophic levels or functional groups. They found that species at the base of the food web are less strongly facilitated, and carnivores are more strongly facilitated, in foundation species' food webs than predicted based on random facilitation, resulting in a higher mean trophic level and a longer average chain length. This indicates foundation species strongly enhance food web complexity through non-trophic facilitation of species across the entire trophic network (Borst et al. 2018).
Overview of Community Structure and Dynamics
Communities are complex entities that can be characterized by their structure (the types and numbers of species present) and dynamics (how communities change over time). Understanding community structure and dynamics enables community ecologists to manage ecosystems more effectively.
Foundation Species
Foundation species are considered the “base” or “bedrock” of a community, having the greatest influence on its overall structure. They are usually the primary producers: organisms that bring most of the energy into the community. Kelp, brown algae, is a foundation species, forming the basis of the kelp forests off the coast of California.
Foundation species may physically modify the environment to produce and maintain habitats that benefit the other organisms that use them. An example is the photosynthetic corals of the coral reef. Corals themselves are not photosynthetic, but harbor symbionts within their body tissues (dinoflagellates called zooxanthellae) that perform photosynthesis; this is another example of a mutualism. The exoskeletons of living and dead coral make up most of the reef structure, which protects many other species from waves and ocean currents.

Biodiversity, Species Richness, and Relative Species Abundance
Biodiversity describes a community’s biological complexity: it is measured in many ways, the simplest of which is by the number of different species (species richness) in a particular area and their relative abundance. Species richness varies across the globe. One factor in determining species richness is latitude, with the greatest species richness occurring in ecosystems near the equator, which often have warmer temperatures, large amounts of rainfall, and low seasonality. The lowest species richness occurs near the poles, which are much colder, drier, and thus less conducive to life in Geologic time (time since glaciations). The predictability of climate or productivity is also an important factor. Other factors influence species richness as well. For example, the study of island biogeography attempts to explain the relatively high species richness found in certain isolated island chains, including the Galápagos Islands that inspired the young Darwin. Foundation species often have the highest relative abundance or biomass of all species in a community.
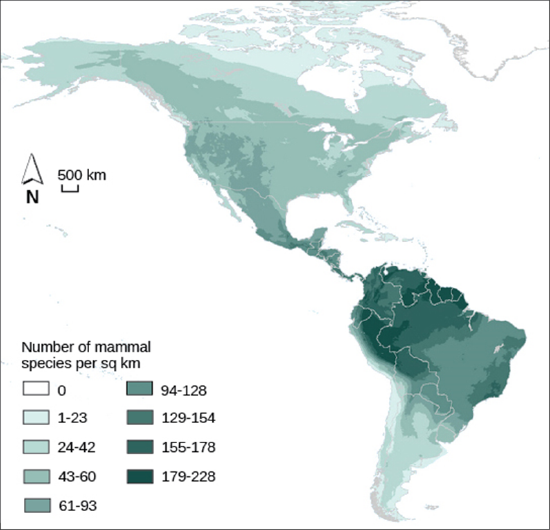
Keystone Species
A keystone species is one whose presence is key to maintaining biodiversity within an ecosystem and to upholding an ecological community’s structure. The intertidal sea star, Pisaster ochraceus, of the northwestern United States is a keystone species. Studies have shown that when this organism is removed from communities, populations of their natural prey (mussels) increase, completely altering the species composition and reducing biodiversity. Another keystone species is the banded tetra, a fish in tropical streams, which supplies nearly all of the phosphorus, a necessary inorganic nutrient, to the rest of the community. If these fish were to become extinct, the community would be greatly affected.

Community Dynamics
Community dynamics are the changes in community structure and composition over time. Sometimes these changes are induced by environmental disturbances such as volcanoes, earthquakes, storms, fires, and climate change. Communities with a stable structure are said to be at equilibrium. Following a disturbance, the community may or may not return to the equilibrium state.
Succession describes the sequential appearance and disappearance of species in a community over time. In primary succession, newly exposed or newly formed land is colonized by living things; in secondary succession, part of an ecosystem is disturbed and remnants of the previous community remain.

Invasive species are non-native organisms that, when introduced to an area out of their native range, threaten the ecosystem balance of that habitat. Many such species exist in the United States, as shown in Figure 14 below. Whether enjoying a forest hike, taking a summer boat trip, or simply walking down an urban street, you have likely encountered an invasive species.

Figure 14: In the United States, invasive species like (a) purple loosestrife (Lythrum salicaria) and the (b) zebra mussel (Dreissena polymorpha) threaten certain aquatic ecosystems. Some forests are threatened by the spread of (c) common buckthorn (Rhamnus cathartica), (d) garlic mustard (Alliaria petiolata), and (e) the emerald ash borer (Agrilus planipennis). The (f) European starling (Sturnus vulgaris) may compete with native bird species for nest holes. (credit a: modification of work by Liz West; credit b: modification of work by M. McCormick, NOAA; credit c: modification of work by E. Dronkert; credit d: modification of work by Dan Davison; credit e: modification of work by USDA; credit f: modification of work by Don DeBold)
One of the many recent proliferations of an invasive species concerns the growth of Asian carp populations. Asian carp were introduced to the United States in the 1970s by fisheries and sewage treatment facilities that used the fish’s excellent filter feeding capabilities to clean their ponds of excess plankton. Some of the fish escaped, however, and by the 1980s they had colonized many waterways of the Mississippi River basin, including the Illinois and Missouri Rivers.
Voracious eaters and rapid reproducers, Asian carp may outcompete native species for food, potentially leading to their extinction. For example, black carp are voracious eaters of native mussels and snails, limiting this food source for native fish species. Silver carp eat plankton that native mussels and snails feed on, reducing this food source by a different alteration of the food web. In some areas of the Mississippi River, Asian carp species have become the most predominant, effectively outcompeting native fishes for habitat. In some parts of the Illinois River, Asian carp constitute 95 percent of the community's biomass. Although edible, the fish is bony and not a desired food in the United States. Moreover, their presence threatens the native fish and fisheries of the Great Lakes, which are important to local economies and recreational anglers. Asian carp have even injured humans. The fish, frightened by the sound of approaching motorboats, thrust themselves into the air, often landing in the boat or directly hitting the boaters.
The Great Lakes and their prized salmon and lake trout fisheries are also being threatened by these invasive fish. Asian carp have already colonized rivers and canals that lead into Lake Michigan. One infested waterway of particular importance is the Chicago Sanitary and Ship Channel, the major supply waterway linking the Great Lakes to the Mississippi River. To prevent the Asian carp from leaving the canal, a series of electric barriers have been successfully used to discourage their migration; however, the threat is significant enough that several states and Canada have sued to have the Chicago channel permanently cut off from Lake Michigan. Local and national politicians have weighed in on how to solve the problem, but no one knows whether the Asian carp will ultimately be considered a nuisance, like other invasive species such as the water hyacinth and zebra mussel, or whether it will be the destroyer of the largest freshwater fishery of the world.
The issues associated with Asian carp show how population and community ecology, fisheries management, and politics intersect on issues of vital importance to the human food supply and economy. Socio-political issues like this make extensive use of the sciences of population ecology (the study of members of a particular species occupying a particular area known as a habitat) and community ecology (the study of the interaction of all species within a habitat).
Populations of species do not exist in isolation – they exist in the context of coevolved ecological communities. Species interactions can harm both species involved (competition), benefit both involved (mutualism), or benefit one and harm the other (Predation, Parasitism, Herbivory). These interactions play a key role in evolution (e.g., prey evolve to avoid detection by predators) and in structuring ecological communities (e.g., loss of top predators can lead to drastic reductions in biodiversity). While some of these interactions involved trophic relationships, others, such as habitat-formation by foundation species, do not.
References
"Predator & Prey: Adaptations" (PDF). Royal Saskatchewan Museum. 2012. Archived from the original (PDF) on 3 April 2018. Retrieved 19 April 2018.
"Types of Pollination, Pollinators and Terminology". CropsReview.Com. Retrieved 2015-10-20.
Angelini C, Altieri AH, Silliman BR, Bertness MD. Interactions among foundation species and their consequences for community organization, biodiversity, and conservation. Bioscience. 2011;61(10):782–9.
Angelini C, Silliman BR. Secondary foundation species as drivers of trophic and functional diversity: evidence from a tree epiphyte system. Ecology. 2014;95(1):185–96. pmid:24649658
Bar-Yam. "Predator-Prey Relationships". New England Complex Systems Institute. Retrieved 7 September 2018.
Begon, M., J.L. Harper and C.R. Townsend. 1996. Ecology: individuals, populations, and communities, Third Edition. Blackwell Science Ltd., Cambridge, Massachusetts, USA.
Bengtson, S. (2002). "Origins and early evolution of predation". In Kowalewski, M.; Kelley, P. H. (eds.). The fossil record of predation. The Paleontological Society Papers 8 (PDF). The Paleontological Society. pp. 289–317.
Bertness MD, Callaway R. Positive interactions in communities. Trends in Ecology & Evolution. 1994;9(5):191–3.
Bertness MD, Leonard GH, Levine JM, Schmidt PR, Ingraham AO. Testing the relative contribution of positive and negative interactions in rocky intertidal communities. Ecology. 1999;80(8):2711–26.
Borst, A.C., Verberk, W.C., Angelini, C., Schotanus, J., Wolters, J.W., Christianen, M.J., van der Zee, E.M., Derksen-Hooijberg, M. and van der Heide, T. (2018) "Foundation species enhance food web complexity through non-trophic facilitation". PLOS ONE, 13(8): e0199152. doi:10.1371/journal.pone.0199152. Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Bruno JF, Stachowicz JJ, Bertness MD. Inclusion of facilitation into ecological theory. Trends in Ecology & Evolution. 2003;18(3):119–25.
Bulleri F, Bruno JF, Silliman BR, Stachowicz JJ. Facilitation and the niche: implications for coexistence, range shifts and ecosystem functioning. Functional Ecology. 2016;30(1):70–8.
Dayton PK. Toward an understanding of community resilience and the potential effects of enrichments to the benthos at McMurdo Sound, Antarctica. In: Parker B, editor. Proceedings of the Colloquium on Conservation Problems in Antarctica. Lawrence, Kansas: Allen Press; 1972.
Ehrlich, Paul R.; Raven, Peter H. (1964). "Butterflies and Plants: A Study in Coevolution". Evolution. 18 (4): 586–608. doi:10.2307/2406212. JSTOR 2406212.
Ellison AM, Bank MS, Clinton BD, Colburn EA, Elliott K, Ford CR, et al. Loss of foundation species: consequences for the structure and dynamics of forested ecosystems. Frontiers in Ecology and the Environment. 2005;3(9):479–86.
Filazzola A, Westphal M, Powers M, Liczner AR, Woollett DA, Johnson B, et al. Non-trophic interactions in deserts: Facilitation, interference, and an endangered lizard species. Basic and Applied Ecology. 2017;20:51–61.
Gómez, José M.; González-Megías, Adela (2002). "Asymmetrical interactions between ungulates and phytophagous insects: Being different matters". Ecology. 83 (1): 203–11. doi:10.1890/0012-9658(2002)083[0203:AIBUAP]2.0.CO;2.
Govenar B. Shaping vent and seep communities: Habitat provision and modification by foundation species. In: Kiel S, editor. The Vent and Seep Biota. Topics in Geobiology. 33: Springer Netherlands; 2010. p. 403–32.
Hardin, Garrett (1960). "The competitive exclusion principle" (PDF). Science. 131 (3409): 1292–1297. Bibcode:1960Sci...131.1292H. doi:10.1126/science.131.3409.1292. PMID 14399717.
Jeppesen E, Sondergaard M, Sondergaard M, Christofferson K. The structuring role of submerged macrophytes in lakes. New York: Springer; 1992.
Jones CG, Gutierrez JL, Byers JE, Crooks JA, Lambrinos JG, Talley TS. A framework for understanding physical ecosystem engineering by organisms. Oikos. 2010;119(12):1862–9.
Kefi S, Berlow EL, Wieters EA, Joppa LN, Wood SA, Brose U, et al. Network structure beyond food webs: mapping non-trophic and trophic interactions on Chilean rocky shores. Ecology. 2015;96(1):291–303. pmid:26236914.
Lim, Ganges; Burns, Kevin C. (2021-11-24). "Do fruit reflectance properties affect avian frugivory in New Zealand?". New Zealand Journal of Botany: 1–11. doi:10.1080/0028825X.2021.2001664. ISSN 0028-825X. S2CID 244683146.
Lunau, Klaus (2004). "Adaptive radiation and coevolution — pollination biology case studies". Organisms Diversity & Evolution. 4 (3): 207–224. doi:10.1016/j.ode.2004.02.002.
Martin, Bradford D.; Schwab, Ernest (2013). "Current usage of symbiosis and associated terminology". International Journal of Biology. 5 (1): 32–45. doi:10.5539/ijb.v5n1p32.
Pocheville, Arnaud (2015). "The Ecological Niche: History and Recent Controversies". In Heams, Thomas; Huneman, Philippe; Lecointre, Guillaume; et al. (eds.). Handbook of Evolutionary Thinking in the Sciences. Dordrecht: Springer. pp. 547–586. ISBN 978-94-017-9014-7.
Pollan, Michael (2001). The Botany of Desire: A Plant's-eye View of the World. Bloomsbury. ISBN 978-0-7475-6300-6.
Poulin, Robert (2007). Evolutionary Ecology of Parasites. Princeton University Press. pp. 4–5. ISBN 978-0-691-12085-0.
Reid AM, Lortie CJ. Cushion plants are foundation species with positive effects extending to higher trophic levels. Ecosphere. 2012;3(11).
Sahney, Sarda; Benton, Michael J.; Ferry, Paul A. (23 August 2010). "Links between global taxonomic diversity, ecological diversity and the expansion of vertebrates on land". Biology Letters. 6 (4): 544–547. doi:10.1098/rsbl.2009.1024. PMC 2936204. PMID 20106856.
Sanders D, Jones CG, Thébault E, Bouma TJ, Heide Tvd, Belzen Jv, et al. Integrating ecosystem engineering and food webs. Oikos. 2014;123(5):513–24.
Toepfer, G. "Amensalism". In: BioConcepts. link.
van der Zee EM, Angelini C, Govers LL, Christianen MJA, Altieri AH, van der Reijden KJ, et al. How habitat-modifying organisms structure the food web of two coastal ecosystems. Proceedings of the Royal Society B-Biological Sciences. 2016;283(1826). pmid:26962135.
van der Zee EM, Tielens E, Holthuijsen S, Donadi S, Eriksson BK, van der Veer HW, et al. Habitat modification drives benthic trophic diversity in an intertidal soft-bottom ecosystem. Journal of Experimental Marine Biology and Ecology. 2015;465:41–8.
Vermeij, Geerat J. (1993). Evolution and Escalation: An Ecological History of Life. Princeton University Press. pp. 11 and passim. ISBN 978-0-691-00080-0.
Willey, Joanne M.; Sherwood, Linda M.; Woolverton, Cristopher J. (2013). Prescott's Microbiology (9th ed.). pp. 713–38. ISBN 978-0-07-751066-4.
Williams E, Mignucci, Williams L & Bonde (November 2003). "Echeneid-sirenian associations, with information on sharksucker diet". Journal of Fish Biology. 5 (63): 1176–1183. doi:10.1046/j.1095-8649.2003.00236.x. Retrieved 17 June 2020.
Wootton, JT; Emmerson, M (2005). "Measurement of Interaction Strength in Nature". Annual Review of Ecology, Evolution, and Systematics. 36: 419–44. doi:10.1146/annurev.ecolsys.36.091704.175535. JSTOR 30033811.
Attributions
This chapter was written by N. Gownaris, A. Howard, C. Olmstead, and T. Zallek, with text taken from the following CC-BY resources:


