Section 21.3: Antibodies
- Page ID
- 146445
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Antibodies
Antibodies (also called immunoglobulins) are glycoproteins that are present in both the blood and tissue fluids. The basic structure of an antibody monomer consists of four protein chains held together by disulfide bonds (Figure \(\PageIndex{4}\)). A disulfide bond is a covalent bond between the sulfhydryl R groups found on two cysteine amino acids. The two largest chains are identical to each other and are called the heavy chains. The two smaller chains are also identical to each other and are called the light chains. Joined together, the heavy and light chains form a basic Y-shaped structure.
The two ‘arms’ of the Y-shaped antibody molecule are known as the Fab region, for “fragment of antigen binding.” The far end of the Fab region is the variable region, which serves as the site of antigen binding. The amino acid sequence in the variable region dictates the three-dimensional structure, and thus the specific three-dimensional epitope to which the Fab region is capable of binding. Although the epitope specificity of the Fab regions is identical for each arm of a single antibody molecule, this region displays a high degree of variability between antibodies with different epitope specificities. Binding to the Fab region is necessary for neutralization of pathogens, agglutination or aggregation of pathogens, and antibody-dependent cell-mediated cytotoxicity.
The constant region of the antibody molecule includes the trunk of the Y and lower portion of each arm of the Y. The trunk of the Y is also called the Fc region, for “fragment of crystallization,” and is the site of complement factor binding and binding to phagocytic cells during antibody-mediated opsonization.
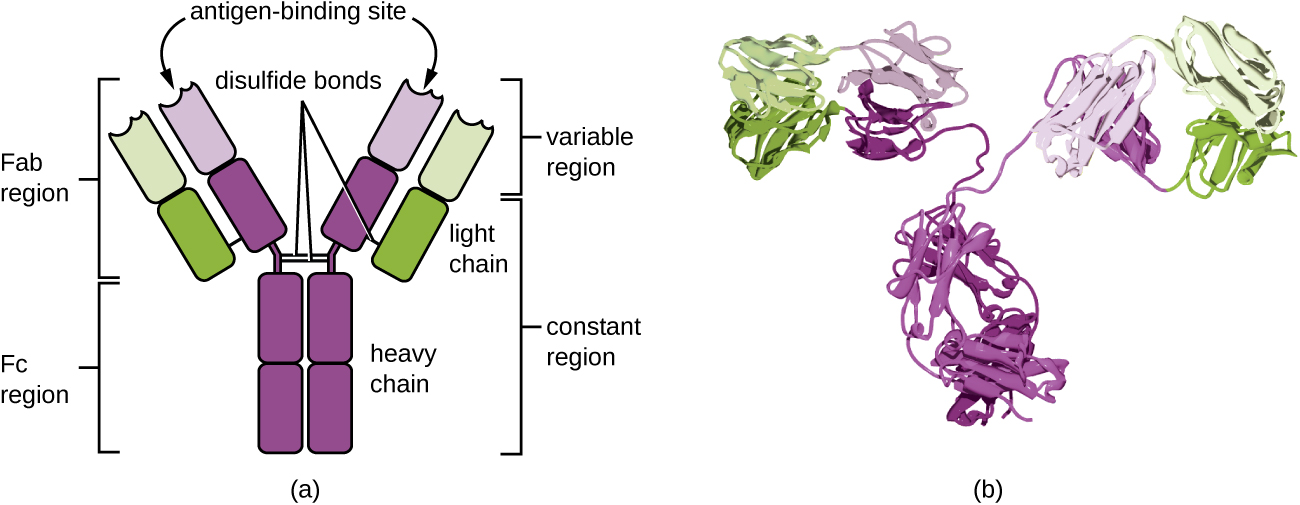
Describe the different functions of the Fab region and the Fc region.
Antibody Classes
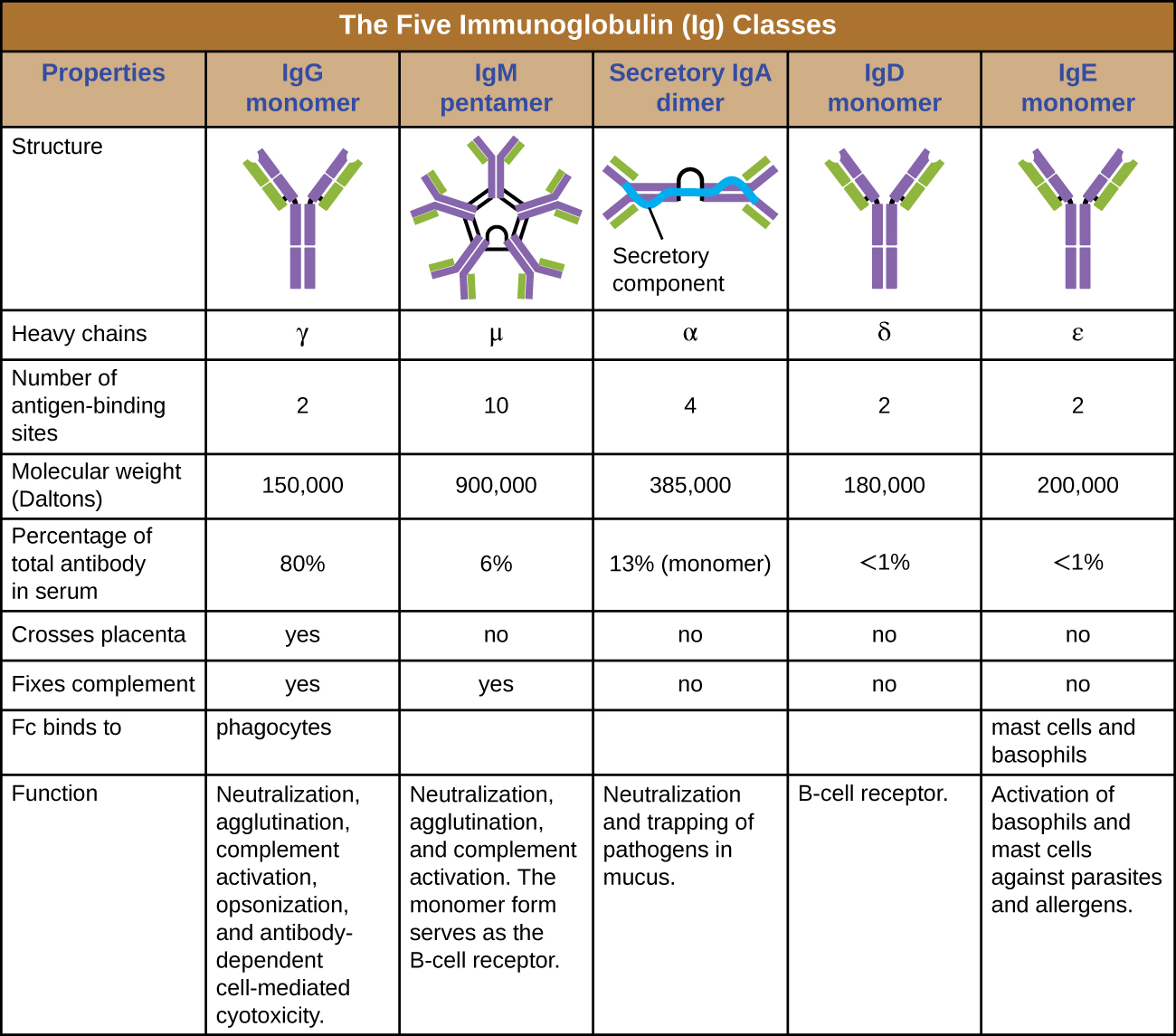
The constant region of an antibody molecule determines its class, or isotype. The five classes of antibodies are IgG, IgM, IgA, IgD, and IgE. Each class possesses unique heavy chains designated by Greek letters γ, μ, α, δ, and ε, respectively. Antibody classes also exhibit important differences in abundance in serum, arrangement, body sites of action, functional roles, and size (Figure \(\PageIndex{5}\)).
IgG is a monomer that is by far the most abundant antibody in human blood, accounting for about 80% of total serum antibody. IgG penetrates efficiently into tissue spaces, and is the only antibody class with the ability to cross the placental barrier, providing passive immunity to the developing fetus during pregnancy. IgG is also the most versatile antibody class in terms of its role in the body’s defense against pathogens.
IgM is initially produced in a monomeric membrane-bound form that serves as an antigen-binding receptor on B cells. The secreted form of IgM assembles into a pentamer with five monomers of IgM bound together by a protein structure called the J chain. Although the location of the J chain relative to the Fc regions of the five monomers prevents IgM from performing some of the functions of IgG, the ten available Fab sites associated with a pentameric IgM make it an important antibody in the body’s arsenal of defenses. IgM is the first antibody produced and secreted by B cells during the primary and secondary immune responses, making pathogen-specific IgM a valuable diagnostic marker during active or recent infections.
IgA accounts for about 13% of total serum antibody, and secretory IgA is the most common and abundant antibody class found in the mucus secretions that protect the mucous membranes. IgA can also be found in other secretions such as breast milk, tears, and saliva. Secretory IgA is assembled into a dimeric form with two monomers joined by a protein structure called the secretory component. One of the important functions of secretory IgA is to trap pathogens in mucus so that they can later be eliminated from the body.
Similar to IgM, IgD is a membrane-bound monomer found on the surface of B cells, where it serves as an antigen-binding receptor. However, IgD is not secreted by B cells, and only trace amounts are detected in serum. These trace amounts most likely come from the degradation of old B cells and the release of IgD molecules from their cytoplasmic membranes.
IgE is the least abundant antibody class in serum. Like IgG, it is secreted as a monomer, but its role in adaptive immunity is restricted to anti-parasitic defenses. The Fc region of IgE binds to basophils and mast cells. The Fab region of the bound IgE then interacts with specific antigen epitopes, causing the cells to release potent pro-inflammatory mediators. The inflammatory reaction resulting from the activation of mast cells and basophils aids in the defense against parasites, but this reaction is also central to allergic reactions (see Diseases of the Immune System.)
- What part of an antibody molecule determines its class?
- What class of antibody is involved in protection against parasites?
- Describe the difference in structure between IgM and IgG.
Key Concepts and Summary
- Adaptive immunity is an acquired defense against foreign pathogens that is characterized by specificity and memory. The first exposure to an antigen stimulates a primary response, and subsequent exposures stimulate a faster and strong secondary response.
- Adaptive immunity is a dual system involving humoral immunity (antibodies produced by B cells) and cellular immunity (T cells directed against intracellular pathogens).
- Antigens, also called immunogens, are molecules that activate adaptive immunity. A single antigen possesses smaller epitopes, each capable of inducing a specific adaptive immune response.
- An antigen’s ability to stimulate an immune response depends on several factors, including its molecular class, molecular complexity, and size.
- Antibodies (immunoglobulins) are Y-shaped glycoproteins with two Fab sites for binding antigens and an Fc portion involved in complement activation and opsonization.
- The five classes of antibody are IgM, IgG, IgA, IgE, and IgD, each differing in size, arrangement, location within the body, and function. The five primary functions of antibodies are neutralization, opsonization, agglutination, complement activation, and antibody-dependent cell-mediated cytotoxicity (ADCC).


