17.3: Ketone Bodies
- Page ID
- 15027
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)-
Describe the Structures and Types of Ketone Bodies:
- Identify and draw the structures of acetoacetate, D-β-hydroxybutyrate, and acetone.
- Explain the chemical differences between these molecules, including their pKa values, and how these properties influence their physiological roles.
-
Understand the Conditions Favoring Ketone Body Formation:
- Explain how high concentrations of acetyl-CoA, derived from fatty acid β‑oxidation, promote ketogenesis in the liver.
- Describe metabolic conditions that lead to ketone body synthesis, such as fasting, starvation, and diabetes, particularly when carbohydrate metabolism is compromised.
-
Outline the Pathways of Ketogenesis and Ketolysis:
- Summarize the key reactions and enzymes involved in ketogenesis (e.g., thiolase, HMG-CoA synthase, and HMG-CoA lyase) and how these reactions lead to the production of ketone bodies.
- Describe ketolysis and how ketone bodies are converted back into acetyl-CoA for entry into the citric acid cycle in extrahepatic tissues.
-
Explain Hormonal Regulation of Ketone Metabolism:
- Discuss the role of insulin, glucagon, cortisol, and catecholamines in modulating fatty acid mobilization, ketogenesis, and ketolysis.
- Analyze how a low-insulin state triggers increased fatty acid oxidation and ketone body production, and how hormonal signals help maintain energy balance during fasting.
-
Evaluate the Role of Ketone Bodies in Energy Homeostasis:
- Explain why ketone bodies are critical for brain energy supply when fatty acids cannot cross the blood-brain barrier.
- Discuss the advantages of ketone bodies as an alternative fuel source during prolonged fasting or in diabetic states.
-
Relate Ketone Body Metabolism to Clinical Conditions:
- Define and contrast nutritional ketosis (as seen with ketogenic diets) versus diabetic ketoacidosis, noting the underlying biochemical mechanisms and clinical consequences.
- Describe the symptoms and metabolic disturbances associated with diabetic ketoacidosis and how they relate to impaired glucose utilization and increased ketone body production.
-
Integrate Fatty Acid Oxidation with Ketone Body Synthesis:
- Explain the biochemical link between fatty acid oxidation, the accumulation of acetyl-CoA, and the activation of ketogenesis in the liver.
- Discuss how changes in metabolic flux through the citric acid cycle (due to depletion of intermediates for gluconeogenesis) promote ketone body production.
These learning goals aim to ensure that students not only understand the molecular mechanisms underlying ketone body synthesis and utilization but also appreciate the broader physiological and clinical contexts in which these processes operate.
Much of the material below derives from Kolb et al. BMC Medicine (2021) 19:313 https://doi.org/10.1186/s12916-021-02185-0. Creative Commons Attribution 4.0 International License, http://creativecommons.org/licenses/by/4.0/.
Introduction
Ketone bodies are the name given to two molecules, acetoacetate (a ketone) and D-β-hydroxybutyrate (its reduction product), that derive from the condensation of acetate in the form of acetyl-CoA. Their structures are shown in Figure \(\PageIndex{1}\).
They are synthesized when acetyl-CoA concentrations are high. That would occur under a couple of conditions:
- fatty acids are being mobilized in adipocytes and broken down into acetyl-CoA through β-oxidation in the mitochondria;
- the acetyl-CoA produced can not enter the Krebs cycle, leading to the buildup of acetyl-CoA in the matrix.
The latter condition arises when carbohydrate metabolism is compromised, slowing the citric acid cycle. This occurs when the citric acid cycle intermediate oxaloacetate is diverted from the cycle for the gluconeogenic synthesis of glucose. Pyruvate, another source of acetyl-CoA (through pyruvate dehydrogenase), is also depleted in gluconeogenesis. Of course, we are describing conditions in the liver, the major site of gluconeogenesis and ketone body synthesis.
These conditions are also met in the underfed or starving state, when glycogen supplies are exhausted and fatty acid reserves are utilized for energy. Likewise, these conditions are met in diabetes when glucose might be abundant in the circulation, but its entry into the cell is compromised by problems with insulin signaling.
Fatty acids released by adipocytes into the blood are bound to serum albumin for transport to tissues. However, with its bound fatty acids, albumin does not cross the blood-brain barrier. Ketone bodies are small and water-soluble, allowing them to cross the blood-brain barrier and deliver a soluble equivalent of fatty acids to the brain and other tissues during times of energy need. Ketone bodies can be considered solubilized and easily transportable equivalents of fatty acids. They have low pKa values of 3.6 (acetoacetate) and 4.7 (D-β-hydroxybutyrate), so they can lead to "ketoacidosis" in diabetics. Acetone is a non-acidic, sweet-smelling molecule that can be detected in the breath of diabetics. It is produced spontaneously and also catalytically through the decarboxylation of the beta-keto acid acetoacetate. It is also considered a ketone body.
The blood-brain barrier (BBB) protects the entire nervous system (CNS). Endothelial cells line the surface of all blood vessels. In the CNS, additional structures prevent the entry of many molecules. Tight and adherence junctions form between endothelial cells, preventing the movement of solutes between the cells. The basement membrane, glial cells, and pericytes provide additional protection. Additionally, drug efflux transporters (ABC transporters) facilitate the movement of xenobiotics (foreign toxic molecules) back across the barrier.
Nonpolar molecules like dioxygen and low-molecular-weight nonpolar molecules can cross the BBB by simple diffusion. Other polar molecules, such as glucose and amino acids, are moved across by transporters. Larger substances can move in through receptor-mediated endocytosis. Albumin, the major carrier of free fatty acids, like other proteins, does not readily pass through the barrier. Neither do albumin:ligand complexes.
Figure \(\PageIndex{2}\) represents the blood-brain barrier.
Figure \(\PageIndex{2}\): Structure of the blood-brain barrier. Wilhelm et al. Int. J. Mol. Sci. 2013, 14, 1383-1411; doi:10.3390/ijms14011383. Creative Commons Attribution License
(http://creativecommons.org/licenses/by/3.0/).
In this section, we will explore the synthesis and utilization of ketone bodies. The synthesis pathway is known as ketogenesis, and the degradation pathway is referred to as ketolysis. Ketone body production and utilization are closely tied to fatty acid metabolism, making it appropriate to discuss them immediately after fatty acid oxidation. An overview of both ketogenesis and ketolysis is shown in Figure \(\PageIndex{3}\).
Figure \(\PageIndex{3}\): Overview of the ketogenesis (left) and ketolysis (right) pathways. FFA, free fatty acids; mThiolase,
mitochondrial thiolase; HMGCS2, hydroxy methylglutaryl-CoA synthase; HMGCL, HMG-CoA lyase; BDH1, mitochondrial βOHB dehydrogenase; MCT1/2, monocarboxylate transporter 1 and 2; SCOT, succinyl-CoA:3-oxoacid-CoA transferase; CS, citrate synthase. Kolb et al. BMC Medicine (2021) 19:313
https://doi.org/10.1186/s12916-021-02185-0 Creative Commons Attribution 4.0 International License. http://creativecommons.org/licenses/by/4.0/.
Most acetoacetate is converted to its reduction product, β-hydroxybutyrate, for blood transport. It can be spontaneously or enzymatically converted to acetone, as it is a β-ketoacid with a built-in electron sink that stabilizes the negative charge in the transition state and intermediate. When ketone bodies enter cells, the β-hydroxybutyrate is converted back to acetoacetate and eventually to acetyl-CoA.
Ketogenesis: The details
Figure \(\PageIndex{4}\) shows the chemical structures of all intermediates in synthesizing ketone bodies.
We'll study three of the reactions in ketogenesis in more detail below.
Reaction 1: Thiolase
We have presented the mechanism of thiolase in Step 4 of mitochondrial β-oxidation of fatty acids using the enzyme acetyl-CoA acetyltransferase (ACAT1), also known as 3-ketoacyl-CoA thiolase. The final step in the beta-oxidation pathway involves cleavage of the bond between the alpha and beta carbon by CoASH. This step is catalyzed by beta-keto thiolase and is a thiolytic reaction (as opposed to cleavage by water, a hydrolysis reaction). The reaction produces one molecule of acetyl-CoA and a fatty acyl-CoA that is two carbons shorter. The process repeats until the even-chain fatty acid is completely converted into acetyl-CoA. The activity of the enzyme is reversible, and it can also catalyze the Claisen condensation of two acetyl-CoA molecules into acetoacetyl-CoA. This reaction occurs in the first step of ketogenesis. This step makes a C-C bond.
Reaction 2: HMG-CoA Synthase
This enzyme catalyzes the condensation of acetoacetyl–CoA (AcAc-CoA) and acetyl–CoA (Ac-CoA) to form 3-hydroxy-3-methylglutaryl (HMG)-CoA, through the formation of a C-C bond. Forming C-C bonds is critical to all biosynthesis. It's not a simple process. As we saw with pyruvate dehydrogenase, cofactors such as thiamine pyrophosphate are often used. The reverse process, breaking a C-C bond, is also difficult unless the atoms are activated. One way to activate a carbon atom is to oxidize it through hydroxylation, often using dioxygen in reactions catalyzed by the cytochrome P450 enzyme. Likewise, C-C bond formation often occurs through the reaction of radicals in hemolytic reactions or through the rearrangement of key ionic intermediates. Radical reactions can occur between a carbon-free radical and heme derivatives. Radical reactions involve heme cofactors (as in P450), non-heme iron proteins (where the iron ion can cycle between different oxidation states), flavoproteins (in which the flavin can undergo both 1 and 2 electron redox steps), radical S-adenosylmethionine (SAM) enzymes, and cobalamins.
HMG-CoA synthase is somewhat unique in forming a C-C bond by activating the methyl group of acetyl-cysteine. The acetyl group comes from an acyl-CoA "donor". The enzyme catalyzes the first committed step in forming complex isoprenoids (like cholesterol) and ketone bodies. The product, 3-hydroxy-3-methylglutaryl HMG–CoA, can either be reduced by HMG-CoA reductase to form mevalonate, which leads to cholesterol synthesis, or cleaved by the enzyme HMG-CoA lyase to produce acetoacetate, a ketone body.
Since it catalyzes the first step in cholesterol synthesis, the enzyme HMG-CoA reductase has been a primary target of drug therapy aimed at reducing high serum cholesterol levels, which are often associated with cardiovascular disease. Over 200 million people worldwide are on statins, the primary drugs used to block HMG-CoA reductase activity. Luckily, statins don't affect HMG-CoA lyase. Gram-positive bacteria also require the mevalonate pathway.
HMG-CoA synthase catalyzes a bisubstrate reaction that displays ping-pong kinetics, which is characteristic of a covalent enzyme intermediate. The first substrate binds to the enzyme and transfers an acetyl group to a nucleophilic Cys 111 in the active site to form an acetyl-Cys 111 intermediate. Free CoA departs. Next, the second substrate, acetoacetyl-CoA, binds and condenses with the acetyl group donated by acetyl-Cys 111. This condensation involves an enolate. Figure \(\PageIndex{5}\) shows a plausible reaction mechanism.
Figure \(\PageIndex{5}\): Reaction mechanism for HMG-CoA synthase
Hence, the reaction consists of three parts: acetylation/deacetylation, condensation/cleavage involving an enolate intermediate, and C-C bond formation and hydrolysis/dehydration. Figure \(\PageIndex{6}\) shows an interactive iCn3D model of the Staphylococcus aureus HMG-COA Synthase with bound HMG-CoA and acetoacetyl-CoA (1XPK)
Figure \(\PageIndex{6}\): Staphylococcus aureus HMG-COA Synthase with bound HMG-CoA and acetoacetyl-CoA (1XPK). (Copyright; author via source). Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...deiFs7JceE5H76
The biologically active unit (homodimer) is shown, with each monomer shown in a different color. The A chain (light cyan) has bound HMG-CoA (HMG) while the B chain (light gold) has acetoacetyl-CoA (CAA) bound. The Glu 79, Cys 111, and His 233 in each subunit are shown in CPK sticks and labeled. Note that Cys 111 is covalently modified in each subunit.
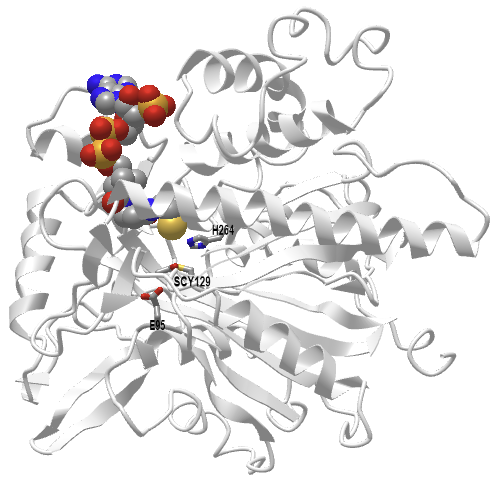
Figure \(\PageIndex{7}\) shows an interactive iCn3D model of the human 3-hydroxy-3-methylglutaryl CoA synthase I with bound CoASH (2P8U)
Figure \(\PageIndex{7}\): Human 3-hydroxy-3-methylglutaryl CoA synthase I (monomer) with bound CoASH (2P8U). (Copyright; author via source). Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...xxEoUfecTk3eL6
The active site side chains are numbered differently (Glu 95, Cys 129, and His 264) than the S. aureus protein. Only the monomer is shown in this model.
Reaction 3: HMG-CoA Lyase
The lyase reaction involves the cleavage of a C-C bond to produce acetyl-CoA and acetoacetate. A gene knockout of this gene in mice is lethal. Figure \(\PageIndex{8}\) shows a plausible mechanism for the reaction.
Figure \(\PageIndex{9}\) shows an interactive iCn3D model of the human HMG-CoA lyase with the competitive inhibitor hydroxyglutaryl-CoA (HG-CoA)(3MP3)
Figure \(\PageIndex{9}\): Human HMG-CoA lyase with inhibitor HG-CoA (3MP3). (Copyright; author via source). Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...VJBWxXpcKQyij8
Only one subunit of the homodimer is shown. The key residues involved in catalysis and binding (Arg41, Asp42, His233, His235, and Cys266) are shown in sticks, with CPK colors and labels. The competitive inhibitor, 3-hydroxyglutaryl-CoA (without the methyl group), is shown in CPK-colored sticks. The S-stereoisomer of HMG-CoA or inhibitor binds, not the R-isomer. Water may shuttle protons between Asp 42 and the C3 hydroxyl of HMG-CoA. Arg 41 facilitates the proper enol/keto tautomer in the enolization of the product. Mutation of Arg 41 drastically reduces catalysis.
We won't discuss the remaining enzymes in detail since we have encountered variants of NAD+/NADH dehydrogenase reactions before. The acetoacetate decarboxylase hastens the already spontaneous decarboxylation of the β-keto ester acetoacetate.
Regulation of Ketogenesis
Hormones such as glucagon, cortisol, thyroid hormones, and catecholamines can upregulate ketogenesis, leading to the mobilization of fatty acids. Insulin is the primary hormonal regulator of this process.
Insulin regulates many key enzymes in the ketogenic pathway, and a state of low insulin triggers the process. A low insulin state leads to:
- Increased free fatty acids (FFAs) arising from decreased inhibition of hormone-sensitive lipase
- Increased uptake of FFAs into the mitochondria arising from decreased activation of acetyl-CoA carboxylase, decreasing malonyl-CoA, which disinhibits Carnitine Palmitoyltransferase 1 (CPT1)
- Increased production of ketone bodies arising from increased HMG-CoA activity
Ketolysis: Utilization of ketone bodies
Figure \(\PageIndex{10}\) shows the chemical steps in using ketone bodies.
Since we have encountered similar reactions, we will not discuss their detailed mechanisms.
Most organs and tissues can utilize ketone bodies as an energy source. They are a major energy source for the brain when glucose is unavailable. That makes sense because fatty acids can't cross the blood-brain barrier. The heart prefers fatty acids for energy but can also use ketone bodies. The liver produces but does not utilize them, as the gene for step 2, β-ketoacyl-CoA transferase, is not transcribed. D-β-hydroxybutyrate is first converted to the other ketone body, acetoacetate, for the pathway to continue. The net result is the formation of two acetyl-CoAs that can then enter the citric acid cycle for ATP production. Acetone is a dead-end ketone body and is excreted in the urine or eliminated in the breath.
Clinical Significance
An overproduction of ketone bodies through increased ketogenesis can cause ketoacidosis (given the pKas of the ketone bodies). One type is diabetic ketoacidosis (DKA) in Type I and II diabetes, which impairs glucose use. This leads to liver gluconeogenesis and the resulting depletion of oxaloacetate and pyruvate, impacting the ability of acetyl-CoA to enter the citric acid cycle.
Upon presentation, patients are often severely dehydrated due to hyperglycemia. High glucose levels in the blood result in increased osmotic pressure in the vessels and decreased water reabsorption in the kidneys. They often have accompanying symptoms like confusion, nausea, vomiting, and abdominal pain. Due to the acidosis, patients often breathe deeply and rapidly to eliminate carbon dioxide, which can lead to respiratory alkalosis. Ketoacidosis can also occur with severe alcoholism and prolonged starvation.
The ketogenic diet, characterized by high-fat and low-carbohydrate consumption, can lead to weight loss. It shifts metabolism to more closely resemble the state of fasting. Its long-term effects are not clear, but some have suggested the use of ketone-based drugs to mimic the effect of ketone bodies. Fasting can also lead to a ketogenic state, and it has been used for the treatment of epilepsy before the drug became available. It is important to differentiate this attempt to induce a ketogenic state from diabetic ketoacidosis.
Summary
This chapter explores the synthesis and utilization of ketone bodies—a critical metabolic adaptation that enables energy production during states of low carbohydrate availability. The key points covered include:
-
Ketone Body Structures and Formation:
The two main ketone bodies, acetoacetate and D-β-hydroxybutyrate, are synthesized in the liver from acetyl-CoA. Acetone, a minor, nonacidic product, is formed spontaneously from acetoacetate. These compounds are produced when acetyl-CoA accumulates, either due to enhanced fatty acid β‑oxidation during fasting, prolonged exercise, or when carbohydrate metabolism is impaired (as in diabetes). -
Regulatory Conditions for Ketogenesis:
Ketone body synthesis is favored under conditions where fatty acids are mobilized from adipose tissue and the citric acid cycle is limited, often due to the withdrawal of oxaloacetate for gluconeogenesis. This metabolic shift ensures that excess acetyl-CoA is diverted to ketogenesis rather than accumulating in the mitochondria. -
Physiological Role and Transport:
Ketone bodies are water soluble and can readily cross the blood–brain barrier, providing an essential energy source for the brain when glucose is scarce. They serve as “solubilized fatty acid equivalents,” ensuring that energy production can continue in vital tissues during prolonged fasting or in diabetic conditions. -
Ketogenesis and Ketolysis Pathways:
The chapter outlines the biochemical pathways of ketogenesis (the formation of ketone bodies) and ketolysis (their utilization). Key enzymes in ketogenesis include thiolase, which catalyzes the reversible condensation of acetyl-CoA molecules, HMG-CoA synthase, which forms 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA), and HMG-CoA lyase, which cleaves HMG-CoA to produce acetoacetate. In ketolysis, ketone bodies are converted back to acetyl-CoA to fuel the citric acid cycle. -
Hormonal Regulation and Metabolic Balance:
Hormonal signals play a central role in controlling ketogenesis. Low insulin levels—typical during fasting or in uncontrolled diabetes—trigger the mobilization of fatty acids and increase ketone body production. Conversely, high insulin levels favor carbohydrate utilization and inhibit ketogenesis. These hormonal cues ensure that ketone body production is finely tuned to meet the organism's energy needs without causing metabolic derangements. -
Clinical Implications:
While ketone bodies serve as an essential alternative energy source, excessive production can lead to ketoacidosis—a dangerous condition characterized by metabolic acidosis. The chapter also distinguishes between nutritional ketosis (as seen with ketogenic diets) and diabetic ketoacidosis, underscoring the clinical importance of balanced ketone metabolism.
In summary, this chapter integrates biochemical pathways with hormonal regulation to illustrate how ketone bodies are synthesized and utilized during energy shortage, highlighting their crucial role in maintaining energy homeostasis and their clinical significance in metabolic diseases.




.png?revision=1&size=bestfit&width=494&height=366)
.png?revision=1&size=bestfit&width=388&height=357)