17.3: Ketone Bodies
- Page ID
- 15027
Much of the material below derives from Kolb et al. BMC Medicine (2021) 19:313 https://doi.org/10.1186/s12916-021-02185-0. Creative Commons Attribution 4.0 International License, http://creativecommons.org/licenses/by/4.0/.
Introduction
Ketone bodies are the name given to two molecules, acetoacetate (a ketone) and D-β-hydroxybutyrate (its reduction product) that derive from the condensation of acetate in the form of acetyl-CoA. Their structures are shown in Figure \(\PageIndex{1}\).
They are synthesized when acetyl-CoA concentrations are high. That would occur under a couple of conditions:
- fatty acids are being mobilized in adipocytes and broken down into acetyl-CoA through β-oxidation in the mitochondria;
- the acetyl-CoA produced can not enter the Kreb cycle leading to the buildup of acetyl-CoA in the matrix.
The latter condition arises when carbohydrate metabolism is compromised so the citric acid cycle has slowed. This occurs if the citric acid cycle intermediate oxaloacetate is pulled away from the cycle for the gluconeogenic synthesis of glucose. Pyruvate, another source of acetyl-CoA (through pyruvate dehydrogenase) is also depleted in gluconeogenesis. Of course, we are describing conditions in the liver, the major site of gluconeogenesis, and also of ketone body synthesis.
These conditions are also met in the underfed or starving state when glycogen supplies are exhausted and fatty acid reserves are being used for energy. Likewise, these conditions are met in diabetes when glucose might be abundant in the circulation but its entry into the cell is compromised by problems with insulin signaling.
Fatty acids released by adipocytes into the blood are bound to serum albumin for transport to tissue. Albumin, with bound fatty acids, does not, however, cross the blood-brain barrier. Ketone bodies are very small and water soluble so they can cross the blood-brain barrier, where they can deliver a soluble equivalent of fatty acids to the brain and other tissues in times of energy need. Ketone bodies can be considered solubilized and easily transportable fatty acid equivalents. They have low pKa values of 3.6 (acetoacetate) and 4.7 (D-β-hydroxybutyrate) so they can lead to "ketoacidosis" in diabetics. Acetone is a nonacidic and sweet-smelling molecule, which can be detected in the breath of diabetics, is produced spontaneously and through catalysis through the decarboxylation of the beta-keto acid acetoacetate. It is also considered a ketone body.
The blood–brain barrier (BBB) protects the entire nervous system (CNS). Endothelial cells line the surface of all blood vessels. In the CNS there are additional structures that prevent the entry of many molecules. Tight and adherence junctions form between endothelial cells that prevent the movement of solutes between the cells. Additional protection is provided by the basement membrane, glial cells, and pericytes. In addition, drug efflux transporters (ABC transporters) move xenobiotics (foreign toxic molecules back through the barrier.
Nonpolar molecules like dioxygen molecules and low molecular weight can cross the BBB by simple diffusion. Other molecules like glucose and amino acids are carried across by transporters. Larger substances can move in through receptor-mediated endocytosis. Albumin, the major carrier of free fatty acids, like other proteins, does not readily pass through the barrier, and its drug-macromolecule complex, cannot cross.
Figure \(\PageIndex{2}\) shows a representation of the blood-brain barrier.
Figure \(\PageIndex{2}\): Structure of the blood-brain barrier. Wilhelm et al. Int. J. Mol. Sci. 2013, 14, 1383-1411; doi:10.3390/ijms14011383. Creative Commons Attribution License
(http://creativecommons.org/licenses/by/3.0/).
In this section, we will explore the synthesis and utilization of ketone bodies. The synthesis pathway is called ketogenesis, and the degradation pathway is called ketolysis. Ketone body production and use are intimately tried to fatty acid metabolism so it is appropriate to discuss them right after fatty acid oxidation. An overview of both ketogenesis and ketolysis is shown in Figure \(\PageIndex{3}\).
Figure \(\PageIndex{3}\): Overview of the ketogenesis (left) and ketolysis (right) pathways. FFA, free fatty acids; mThiolase,
mitochondrial thiolase; HMGCS2, hydroxy methylglutaryl-CoA synthase; HMGCL, HMG-CoA lyase; BDH1, mitochondrial βOHB dehydrogenase; MCT1/2, monocarboxylate transporter 1 and 2; SCOT, succinyl-CoA:3-oxoacid-CoA transferase; CS, citrate synthase. Kolb et al. BMC Medicine (2021) 19:313
https://doi.org/10.1186/s12916-021-02185-0 Creative Commons Attribution 4.0 International License. http://creativecommons.org/licenses/by/4.0/.
Most acetoacetate is converted to its reduction product, β-hydroxybutyrate, for blood transport. It can spontaneously or through enzymatic catalysis, can be converted to acetone since it is a β-ketoacid with a built-in electron sink that stabilizes the negative charge in the transition state and intermediate. When ketone bodies enter cells, the β-hydroxybutyrate is converted back to acetoacetate and eventually to acetyl-CoA.
Ketogenesis: The details
Figure \(\PageIndex{4}\) shows the chemical structures of all intermediates in the synthesis of ketone bodies.
We'll study three of the reactions in ketogenesis in more detail below.
Reaction 1: Thiolase
We have presented the mechanism of thiolase in Step 4 of mitochondrial β-oxidation of fatty acid using the enzyme acetyl-CoA acetyltransferase (ACAT1) also called 3-ketoacyl-CoA thiolase. The final step in the beta-oxidation pathway involves cleavage of the bond between the alpha and beta carbon by CoASH. This step is catalyzed by beta-keto thiolase and is a thiolytic (as opposed to cleavage by water - a hydrolysis) reaction. The reaction produces one molecule of acetyl CoA and a fatty acyl CoA that is two carbons shorter. The process repeats until the even chain fatty acid is completely converted into acetyl CoA. The activity of the enzyme is reversible and it can also catalyze the Claisen condensation of two acetyl-CoA molecules into acetoacetyl-CoA, which occurs in the first step of ketogenesis. This step makes a C-C bond.
Reaction 2: HMG-CoA Synthase
This enzyme catalyzes the condensation of acetoacetyl–CoA (AcAc–CoA) and acetyl–CoA (Ac-CoA) to form 3-hydroxy-3-methylglutaryl (HMG)–CoA, through the formation of a C-C bond. Forming C-C bonds is critical to all biosynthesis. It's not a simple process. Cofactors are often used such as thiamine pyrophosphate, as we saw with pyruvate dehydrogenase. The reverse process, breaking a C-C bond, is also difficult unless the atoms are activated. One way to activate a carbon atom is to oxidize it through hydroxylation, often using dioxygen in reactions catalyzed by cytochrome P450. Likewise, C-C bond formation often occurs through the reaction of radicals in hemolytic reactions or through the rearrangement of key ionic intermediates. Radical reactions can be between a carbon free radical and heme derivatives. Radical reactions involve heme cofactors (as in P450), non-heme iron proteins (where the iron ion can cycle between different oxidation states), flavoproteins (in which the flavin can undergo both 1 and 2 electron redox steps), radical S-adenosylmethionine (SAM) enzymes, and cobalamins.
HMG-CoA synthase is somewhat unique in that it forms a C-C bond by activating the methyl group of acetyl-cysteine. The acetyl group comes from an acyl-CoA "donor". The enzyme catalyzes the first committed step in the formation of complex isoprenoids (like cholesterol) and ketone bodies. The product, 3-hydroxy-3-methylglutaryl HMG–CoA, can either be reduced by HMG-CoA reductase to form mevalonate, which leads to cholesterol synthesis, or cleaved by the enzyme HMG-CoA lyase, to produce acetoacetate, a ketone body.
Since it catalyzes the first step in cholesterol synthesis, the enzyme HMG-CoA reductase has been a primary focus of drug therapy to reduce high levels of serum cholesterol, which is generally associated with cardiovascular disease. Over 200 million people worldwide are on statins, the primary drugs used to block HMG-CoA reductase activity. Luckily, it doesn't affect HMG-CoA lyase. Gram-positive bacteria also require the mevalonate pathway.
HMG-CoA synthase catalyzes a bisubstrate reaction that displays ping-pong kinetics, characteristic of a covalent enzyme intermediate. The first substrate binds to the enzyme and transfers an acetyl group to a nucleophilic Cys 111 in the active site to form an acetyl-Cys 111 intermediae. Free CoA departs. Next the second substrate, acetoacetyl-CoA binds, and condenses with the acetyl group donated by acetyl-Cys 111. This condensation involves an enolate. A plausible reaction mechanism is shown in Figure \(\PageIndex{5}\).
Figure \(\PageIndex{5}\): Reaction mechanism for HMG-CoA synthase
Hence there are three parts of the reaction: acetylation/deacetylation, condensation/cleavage with an enolate intermediate, and C-C formation and hydrolysis/dehydration. Figure \(\PageIndex{6}\) shows an interactive iCn3D model of the Staphylococcus aureus HMG-COA Synthase with bound HMG-CoA and acetoacetyl-CoA (1XPK)
Figure \(\PageIndex{6}\): Staphylococcus aureus HMG-COA Synthase with bound HMG-CoA and acetoacetyl-CoA (1XPK). (Copyright; author via source). Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...deiFs7JceE5H76
The biologically active unit (homodimer) is shown with each monomer shown in a different color. The A chain (light cyan) has bound HMG-CoA (HMG) while the B chain (light gold) has acetoacetyl-CoA (CAA) bound. The Glu 79, Cys 111, and His 233 in each subunit are shown in CPK sticks and labeled. Note that the Cys 111 is covalently modified in each subunit.
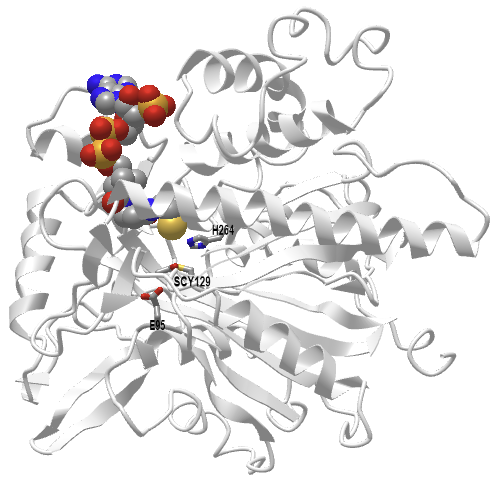
Figure \(\PageIndex{7}\) shows an interactive iCn3D model of the human 3-hydroxy-3-methylglutaryl CoA synthase I with bound CoASH (2P8U)
Figure \(\PageIndex{7}\): Human 3-hydroxy-3-methylglutaryl CoA synthase I (monomer) with bound CoASH (2P8U). (Copyright; author via source). Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...xxEoUfecTk3eL6
The active site side chains are numbered differently (Glu 95, Cys 129, and His 264) compared to the S. aureus protein. Only the monomer is shown in this model.
Reaction 3: HMG-CoA Lyase
The lyase reaction is a C-C bond cleavage to produce acetyl-CoA and acetoacetate. A gene knockout of this gene in mice is lethal. Figure \(\PageIndex{8}\) shows a plausible mechanism for the reaction.
Figure \(\PageIndex{9}\) shows an interactive iCn3D model of the human HMG-CoA lyase with the competitive inhibitor hydroxyglutaryl-CoA (HG-CoA)(3MP3)
Figure \(\PageIndex{9}\): Human HMG-CoA lyase with inhibitor HG-CoA (3MP3). (Copyright; author via source). Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...VJBWxXpcKQyij8
Only one subunit of the homodimer is shown. The key residues involved in catalysis and binding (Arg 41, Asp 42, His 233, His 235, and Cys 266) are shown in sticks, CPK colors, and labeled. The competitive inhibitor, 3-hydroxyglutaryl-CoA (without the methyl group) is shown in CPK-colored sticks. The S-stereoisomer of HMG-CoA or inhibitor binds, not the R-isomer. Water may shuttle protons between Asp 42 and the C3 hydroxyl of HMG-CoA. Arg 41 facilitates the proper enol/keto tautomer of the product mutation on reaction product enolization. Mutation of Arg 41 have lhas effect on catalysis.
We won't go into detail on the remaining enzymes since we have encountered variants of NAD+/NADH dehydrogenase reactions before. The acetoacetate decarboxylase just hastens the already spontaneous decarboxylation of the β-keto ester acetoacetate.
Regulation of Ketogenesis
Ketogenesis can be upregulated by hormones such as glucagon, cortisol, thyroid hormones, and catecholamines which lead to fatty acid mobilization. Insulin is the primary hormonal regulator of this process.
Insulin regulates many key enzymes in the ketogenic pathway, and a state of low insulin triggers the process. A low insulin state leads to:
- Increased free fatty acids (FFAs) arising from decreased inhibition of hormone-sensitive lipase
- Increased uptake of FFAs into the mitochondria arising from decreased activation of acetyl-CoA carboxylase, decreasing malonyl CoA, which disinhibits Carnitine Palmitoyltransferase 1 (CPT1)
- Increased production of ketone bodies arising from increased HMG-CoA activity
Ketolysis: Utilization of ketone bodies
Figure \(\PageIndex{10}\) shows the chemical structures in the utilization of ketone bodies.
Since we have encountered reactions similar to these before, we will not discuss their detailed mechanisms.
Most organs and tissues can use ketone bodies for energy. They are a major source of energy for the brain when glucose is not available. That makes sense given that fatty acids can't cross the blood-brain barrier. The heart prefers fatty acids for energy but can also use ketone bodies. The liver makes but does not use them since the gene for step 2, beta ketoacyl-CoA transferase is not transcribed. D-β-hydroxybutyrate is first converted to the other ketone body, acetoacetate, for the pathway to continue. The net result is the formation of two acetyl-CoAs that can then enter the citric acid cycle for ATP production. Acetone is a dead-end ketone body and is excreted in the urine or eliminated in the breath.
Clinical Significance
An overproduction of ketone bodies through increased ketogenesis can cause ketoacidosis (given the pKas of the ketone bodies). One type is diabetic ketoacidosis (DKA) in Type I and II diabetes which impair glucose use. This leads to liver gluconeogenesis and the resulting depletion of oxaloacetate and pyruvate, impacting the ability of acetyl-CoA to enter the citric acid cycle.
On presentation, patients are usually very dehydrated from being hyperglycemic. High glucose levels in the blood cause higher osmotic pressure in the vessels and decreased water reabsorption in the kidneys. They often have accompanying symptoms like confusion, nausea, vomiting, and abdominal pain. Because of the acidosis, patients often breathe very deeply and rapidly to eliminate carbon dioxide, which can cause and cause respiratory alkalosis. Ketoacidosis also can occur with severe alcoholism and prolonged starvation.
The ketogenic diet, characterized by high fat and low carbohydrate consumption, can lead to weight loss. It shifts metabolism to more closely resemble the fasting state. Its long-term effects are not clear but some have suggested the use of ketone-based drugs to mimic the effect of ketone bodies. Fasting can also lead to a ketogenic state and it has been used for the treatment of epilepsy before the drug became available. It is important to differentiate this attempt to induce a ketogenic state from diabetic ketoacidosis.




.png?revision=1&size=bestfit&width=494&height=366)
.png?revision=1&size=bestfit&width=388&height=357)