5.5A: An Overview to Resisting Innate Immune Defenses
- Page ID
- 3165
- Describe the following as they relate to phagocytosis:
- unenhanced attachment
- enhanced attachment
- ingestion
- destruction
- State 4 different body defense functions of the body's complement pathways.
- State what is meant by antibacterial peptides and give an example.
An Overview of Phagocytosis
As will be seen in Unit 5, there are several steps involved in phagocytosis.
a. Attachment
First the surface of the microbe must be attached to the cytoplasmic membrane of the phagocyte. Attachment of microorganisms is necessary for ingestion and may be unenhanced or enhanced.
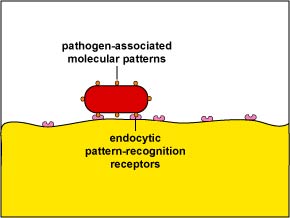
1. Unenhanced attachment is a general recognition of what are called pathogen-associated molecular patterns or PAMPs - components of common molecules such as peptidoglycan, teichoic acids, lipopolysaccharide, mannans, and glucans common in microbial cell walls but not found on human cells - by means of glycoprotein known as endocytic pattern-recognition receptors on the surface of the phagocytes (Figure \(\PageIndex{1}\)).
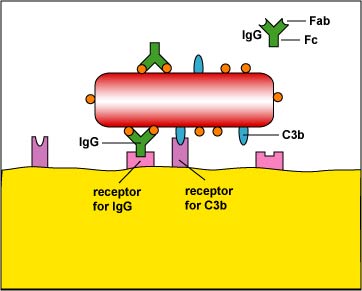
2. Enhanced attachment is the attachment of microbes to phagocytes by way of molecules such as an antibody molecule called IgG and two proteins produced during the complement pathways called C3b and C4b (Figure \(\PageIndex{2}\)). Molecules such as IgG, C3b, and C4b that promote enhanced attachment are called opsonins and the process is called opsonization. Enhanced attachment is much more specific and efficient than unenhanced.
b. Ingestion
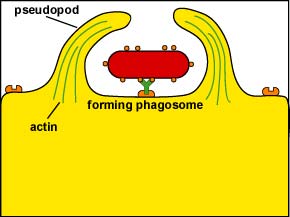
Following attachment, polymerization and then depolymerization of actin filaments send pseudopods out to engulf the microbe (Figure \(\PageIndex{3}\)) and place it in a vesicle called a phagosome (Figure \(\PageIndex{4}\)).
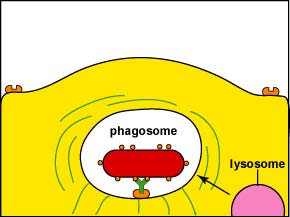
During this process, an electron pump brings hydrogen ions (H+) into the phagosome. This lowers the pH within the phagosome so that when a lysosome fuses with the phagosome, the pH is correct for the acid hydrolases to effectively break down cellular proteins.
c. Destruction
1. Intracellular destruction: Finally, lysosomes, containing digestive enzymes and microbicidal chemicals, fuse with the phagosome containing the ingested microbe and the microbe is destroyed (Figure \(\PageIndex{5}\)).
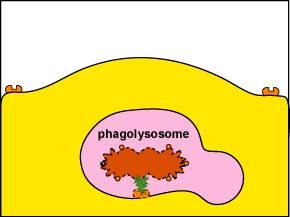
2. Extracellular destruction: If the the infection site contains very large numbers of microorganisms and high levels of inflammatory cytokines and chemokines are being produced in response to PAMPs, the phagocyte will empty the contents of its lysosomes by a process called degranulation to kill the microorganisms or cell extracellularly.
To view a scanning electron micrograph of a macrophage with pseudopods and phagocytosis of E. coli by a macrophage on a blood vessel, see Dennis Kunkel's Microscopy, University of Hawaii-Manoa.
An Overview of the Body's Complement Pathways
Some bacteria are able to interfere with the body's complement pathways. The complement pathways will be discussed in detail later in Unit 4, but a brief summary is relevant here. There are three complement pathways: the classical complement pathway, the alternative complement pathway, and the lectin pathway. While the three pathways differ in the way they are activated, once activated they all produce the same beneficial complement proteins. Basically the complement proteins are a series of serum proteins that when activated participate in four important body defense functions. These include:
a. Inflammation
Inflammation is the means by which body defense cells and defense chemicals leave the blood and enter the tissue around an injured or infected site. Complement proteins known as C5a, C3a, and C4a lead to vasodilation, increased capillary permeability, and the expression of the adhesion molecules on leukocytes and the vascular endothelium. This enables leukocytes to adhere to the inner wall of the capillaries, pass between the endothelial cells, and enter the surrounding tissue. Vasodilation also enables a variety of defense chemicals in the plasma of the blood to enter the tissue. These defense chemicals include antibodies and complement proteins. C5a also causes neutrophils to release proteases and toxic oxygen radicals for extracellular killing of microbes.
b. Phagocyte Chemotaxis
Complement proteins C3a and C4a are chemoattractants for leukocytes. Chemotaxis enables the phagocytes to move toward the infected area in order to remove microorganisms.
c. Opsonization (Enhanced Attachment)
The complement proteins C3b and C4b are known as opsonins because they bind microbes to phagocytes (Figure \(\PageIndex{2}\)). One portion of the molecule binds to microbial proteins while the other portion binds to receptors on phagocytes. In this way, microbes can be engulfed by phagocytes more effectively.
d. MAC Lysis of Biological Membranes
A series of complement proteins known as the membrane attack complex or MAC put pores in cellular membranes resulting in lysis. This is used to kill such things as Gram-negative bacteria, virus-infected cells, and tumor cells.
These processes will be discussed in greater detail in Unit 5.
- Capsules often enable bacteria to resist phagocytosis by unenhanced attachment. Based on what we just learned, explain how.
- Some bacteria are able to inhibits the C3 convertase enzyme, the enzyme that splits complement protein C3 into C3a and C3b. Explain how this might make it harder for that bacterium to be phagocytosed.
Antibacterial Peptides
The body produces a number of antibacterial peptides such as human defensins and cathelicidins that are directly toxic by forming pores in the cytoplasmic membrane of a variety of microorganisms causing leakage of cellular needs. They also activate cells for an inflammatory response. Defensins are produced by leukocytes, epithelial cells, and other cells. They are also found in blood plasma and mucus.
Some bacteria are able to resist phagocytosis and interfere with the body's complement pathways. In the next two sections we will look at the following virulence factors:
- The ability to resist phagocytic engulfment (attachment and ingestion)
- The ability to resist phagocytic destruction and serum lysis
Summary
- For phagocytosis to occur, the surface of the microbe must be attached to the cytoplasmic membrane of the phagocyte through unenhanced or enhanced attachment.
- Following attachment, the microbe must be engulfed and placed on a membrane-bound vesicle called a phagosome. The phagosome then becomes acidified to provide the correct pH for killing by lysosomal enzymes.
- Lysosomes, containing digestive enzymes and microbicidal chemicals, fuse with the phagosome containing the ingested microbe and the microbe is destroyed. This is referred to as intracellular killing by phagocytes and happens when microbial numbers are relatively low.
- If the the infection site contains very large numbers of microorganisms and high levels of inflammatory cytokines and chemokines are being produced, the phagocyte will empty the contents of its lysosomes by a process called degranulation in order to kill the microorganisms extracellularly. This is referred to as extracellular killing.
- The body’s complement pathways consist of a variety of complement proteins that when activated participate in four important body defense functions: promoting inflammation, phagocyte chemotaxis, opsonization (enhanced attachment), and lysis of membrane-bound cells.
- The body produces a number of antibacterial peptides that are directly toxic by forming pores in the cytoplasmic membrane of a variety of microorganisms causing leakage of cellular needs.


