18.11: Endosymbiosis
- Page ID
- 5935
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The endosymbiosis theory postulates that the mitochondria of eukaryotes evolved from an aerobic bacterium (probably related to the rickettsias) living within an archaeal host cell and the chloroplasts of red algae, green algae, and plants evolved from an endosymbiotic cyanobacterium living within a mitochondria-containing eukaryotic host cell.
The Evidence
- Both mitochondria and chloroplasts can arise only from preexisting mitochondria and chloroplasts. They cannot be formed in a cell that lacks them because nuclear genes encode only some of the proteins of which they are made.
- Both mitochondria and chloroplasts have their own genome, and it resembles that of bacteria not that of the nuclear genome.
- Both genomes consist of a single circular molecule of DNA.
- There are no histones associated with the DNA.
- Both mitochondria and chloroplasts have their own protein-synthesizing machinery, and it more closely resembles that of bacteria than that found in the cytoplasm of eukaryotes.
- The first amino acid of their transcripts is always fMet as it is in bacteria (not methionine [Met] that is the first amino acid in eukaryotic proteins).
- A number of antibiotics (e.g., streptomycin) that act by blocking protein synthesis in bacteria also block protein synthesis within mitochondria and chloroplasts. They do not interfere with protein synthesis in the cytoplasm of the eukaryotes.
- Conversely, inhibitors (e.g., diphtheria toxin) of protein synthesis by eukaryotic ribosomes do not - sensibly enough - have any effect on bacterial protein synthesis nor on protein synthesis within mitochondria and chloroplasts.
- The antibiotic rifampicin, which inhibits the RNA polymerase of bacteria, also inhibits the RNA polymerase within mitochondria. It has no such effect on the RNA polymerase within the eukaryotic nucleus.
The Mitochondrial Genome
The genome of human mitochondria contains 16,569 base pairs of DNA organized in a closed circle (Figure \(\PageIndex{1}\)). These encode 2 ribosomal RNA (rRNA), molecules, 22 transfer RNA (tRNA) molecules, and 13 polypeptides. The 13 polypeptides participate in building several protein complexes embedded in the inner mitochondrial membrane and include 7 subunits that make up the mitochondrial NADH dehydrogenase, 3 subunits of cytochrome c oxidase, 2 subunits of ATP synthase, and cytochrome b.
All these gene products are used within the mitochondrion, but the mitochondrion also needs >900 different proteins as well as some mRNAs and tRNAs encoded by nuclear genes. The proteins (e.g., cytochrome c and the DNA polymerases used within the mitochondrion) are synthesized in the cytosol and then imported into the mitochondrion.
The Chloroplast Genome
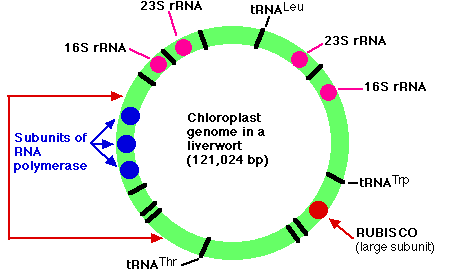
The genome of the chloroplasts found in Marchantia polymorpha (a liverwort, one of the Bryophyta) contains 121,024 base pairs in a closed circle. These make up some 128 genes which include:
- duplicate genes encoding each of the four subunits (23S, 16S, 4.5S, and 5S) of the ribosomal RNA (rRNA) used by the chloroplast
- 37 genes encoding all the transfer RNA (tRNA) molecules used for translation within the chloroplast. Some of these are represented in the figure by black bars (a few of which are labeled).
- 4 genes encoding some of the subunits of the RNA polymerase used for transcription within the chloroplast (3 of them shown in blue)
- a gene encoding the large subunit of the enzyme RUBISCO (ribulose bisphosphate carboxylase oxygenase)
- 9 genes for components of photosystems I and II
- 6 genes encoding parts of the chloroplast ATP synthase
- genes for 19 of the ~60 proteins used to construct the chloroplast ribosome
- All these gene products are used within the chloroplast, but all the chloroplast structures also depend on proteins RUBISCO, for example, the enzyme that adds CO2 to ribulose bisphosphate to start the Calvin cycle, consists of multiple copies of two subunits:
- Encoded by nuclear genes translated in the cytosol, and imported into the chloroplast.
- A large one encoded in the chloroplast genome and synthesized within the chloroplast, and a small subunit encoded in the nuclear genome and synthesized by ribosomes in the cytosol. The small subunit must then be imported into the chloroplast.
- The arrangement of genes shown in the figure is found not only in the Bryophytes (mosses and liverworts) but also in the lycopsids (e.g., Lycopodium and Selaginella). In all other plants, however, the portion of DNA bracketed by the red arrows on the left is inverted. The same genes are present but in inverted order. The figure is based on the work of Ohyama, K., et al., Nature, 322:572, 7 Aug 1986; and Linda A. Raubeson and R. K. Jansen, Science, 255:1697, 27 March 1992.
- The evolution of the eukaryotic chloroplast by the endosymbiosis of a cyanobacterium in a mitochondria-containing eukaryotic host cell led to the evolution of the green algae and plants as described above, red algae, and glaucophytes; a small group of unicellular algae.
Secondary Endosymbiosis: Eukaryotes Engulfing Eukaryotes
The Nucleomorph
Once both heterotrophic and photosynthetic eukaryotes had evolved, the former repeatedly engulfed the latter to exploit their autotrophic way of life. Many animals living today engulf algae for this purpose. Usually the partners in these mutualistic relationships can be grown separately. However, a growing body of evidence indicates that the chloroplasts of some algae have not been derived by engulfing cyanobacteria in a primary endosymbiosis like those discussed above, but by engulfing photosynthetic eukaryotes (Figure 18.11.3). This is called secondary endosymbiosis. It occurred so long ago that these endosymbionts cannot be cultured away from their host.
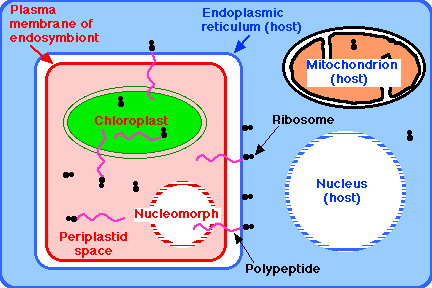
In two groups, the eukaryotic nature of the endosymbiont can be seen by its retention of a vestige of a nucleus (called its nucleomorph). A group of unicellular, motile algae called cryptomonads appear to be the evolutionary outcome of a nonphotosynthetic eukaryotic flagellate (i.e., a protozoan) engulfing a red alga by endocytosis. Another tiny group of unicellular algae, called chlorarachniophytes, appear to be the outcome of a flagellated protozoan having engulfed a green alga.
The result in both cases: a motile, autotrophic cell containing its own nucleus, its own mitochondria, and its own endoplasmic reticulum. The latter of which contains the endosymbiont with:
- its own plasma membrane
- its own cytoplasm, the periplastid space
- its own ribosomes
- its own chloroplast, and
- its nucleomorph - only a vestige of its original nucleus, but still surrounded by a nuclear envelope perforated with nuclear pore complexes and containing a tiny but still-functioning genome.
The Four Genomes of Guillardia theta
The cryptomonad Guillardia theta contains four different genomes:
- its own nuclear genome; by far the largest with 87.2x106 base pairs (bp) of DNA
- the genome of its mitochondria (48,000 bp)
- the genome of the chloroplast in its endosymbiont (121,000 bp)
- the genome of the nucleomorph (551,264 bp)
Susan Douglas and her colleagues reported (in the 26 April 2001 issue of Nature) the completely-sequenced genome of the nucleomorph.
- It contains 3 small chromosomes with
- 47 genes for nonmessenger RNAs (rRNA, tRNA, snRNA)
- 464 genes for messenger RNA; that is, encoding proteins such as
- 65 proteins for its own ribosomes
- 30 for its chloroplast (a small fraction of the hundreds needed)
- a variety of proteins needed within the nucleomorph, including
- DNA licensing factors
- histones
- proteins needed for DNA replication (but no genes for DNA polymerases, which must be translated by and imported from the host ribosomes)
The genes are crowded closely on the three chromosomes. In fact, 44 of them overlap each other. Only 17 genes contain introns, and these are very small.
Genome Interactions in Guillardia theta
Millions of years of evolution have resulted in a complex but precisely-orchestrated array of interactions between the 4 genomes. For example:
- The chloroplast needs proteins synthesized by 3 different genomes: its own, the nucleomorph's, and the host's.
- The nucleomorph genome has given up all (but one) of its genes encoding enzymes for general metabolic functions; the endosymbiont now depends on those encoded by the host nucleus.
- The nucleomorph itself also depends on genes (e.g., for DNA polymerases) residing in the host nucleus.
The Apicoplast
The apicoplast (short for "apicomplexan plastid") is a solitary organelle found in the apicomplexan protists: "sporozoans" like Plasmodium falciparum (and the other agents of malaria) and Toxoplasma gondii.
Features:
- Essential - the organisms cannot survive without it.
- Encased by 4 membranes.
- Contains its own genome, a circular molecule of DNA (35,000 base pairs) which encodes
- ~ 30 proteins
- a full set of tRNAs plus some other RNAs
- Only a few functions have been discovered, but these include
- anabolic metabolism such as the synthesis of fatty acids
- repair, replication, transcription, and translation of its genes
- Clearly 30 proteins are not enough to accomplish so many functions so the apicoplast has to import from the cytosol ~500 nuclear-encoded proteins.
The apicoplast is the product of an ancient endosymbiosis in which the eukaryotic ancestor engulfed a unicellular alga - probably a red alga - with a solitary chloroplast. Over time, the nucleus was lost (no residual nucleomorph) as well as many features of the chloroplast (including its ability to perform photosynthesis).
Two Japanese scientists have discovered a heterotrophic flagellate that engulfs a unicellular green alga that lives freely in the surrounding water. Once inside, the alga loses its flagella and cytoskeleton; the host loses its feeding apparatus. Moreover, the host switches from heterotrophic to autotrophic nutrition (photosynthesis) and the host becomes capable of phototaxis. When the host divides by mitosis, only one daughter cell gets the plastid. The other cell regrows the feeding apparatus and is ready to engulf another alga.


