11.10: Antisense RNA
- Page ID
- 4934
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Messenger RNA (mRNA) is single-stranded. Its sequence of nucleotides is called "sense" because it results in a gene product (protein). Normally, its unpaired nucleotides are "read" by transfer RNA anticodons as the ribosome proceeds to translate the message.
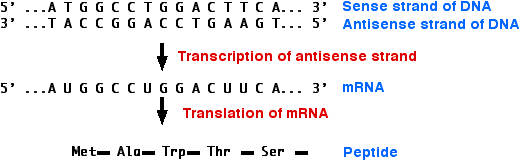
However, RNA can form duplexes just as DNA does. All that is needed is a second strand of RNA whose sequence of bases is complementary to the first strand.
Example
5´ C A U G 3´ mRNA
3´ G U A C 5´ Antisense RNA
The second strand is called the antisense strand because its sequence of nucleotides is the complement of message sense.

When mRNA forms a duplex with a complementary antisense RNA sequence, translation is blocked. This may occur because the ribosome cannot gain access to the nucleotides in the mRNA or because the duplex RNA is quickly degraded by ribonucleases in the cell. With recombinant DNA methods, synthetic genes (DNA) encoding antisense RNA molecules can be introduced into the organism.
Examples
The Flavr Savr tomato
Most tomatoes that have to be shipped to market are harvested before they are ripe. Otherwise, ethylene synthesized by the tomato causes them to ripen and spoil before they reach the customer. Transgenic tomatoes have been constructed that carry in their genome an artificial gene (DNA) that is transcribed into an antisense RNA complementary to the mRNA for an enzyme involved in ethylene production. These tomatoes make only 10% of the normal amount of the enzyme.
The goal of this work was to provide supermarket tomatoes with something closer to the appearance and taste of tomatoes harvested when ripe. However, these tomatoes often became damaged during shipment and handling and have been taken off the market.
Transgenic Tobacco

Fig.11.9.3 tobacco flower
Flower of a tobacco plant carrying a transgene whose transcript is antisense to one of the mRNAs needed for normal flower pigmentation.
Transgenic Flower

Flower of another transgenic plant that failed to have its normal pigmentation altered.
Making transgenic plants
There are several methods for introducing genes into plants, including
- infecting plant cells with plasmid vectors carrying the desired gene
- shooting microscopic pellets containing the gene directly into the cell
In contrast to animals, there is no real distinction between somatic cells and germline cells. Somatic tissues of plants, e.g., root cells grown in culture,
- can be transformed in the laboratory with the desired gene
- grown into mature plants with flowers.
If all goes well, the transgene will be incorporated into the pollen and eggs and passed on to the next generation.
In this respect, it is easier to produce transgenic plants than transgenic animals.
Antisense RNA also occurs naturally
Do cells contain genes that are naturally translated into antisense RNA molecules capable of blocking the translation of other genes in the cell? The answer is yes, and these seem to represent another method of regulating gene expression. In both mice and humans, the gene for the insulin-like growth factor 2 receptor (Igf2r) that is inherited from the father synthesizes an antisense RNA that appears to block synthesis of the mRNA for Igf2r. An inherited difference in the expression of a gene depending on whether it is inherited from the mother or the father is called genomic or parental imprinting.
RNA interference (RNAi)
In testing the effects of antisense RNA, one should use sense RNA of the same coding region as a control. Surprisingly, preparations of sense RNA often turn out to be as effective an inhibitor as antisense RNA.
Why? It seems that the preparations of sense RNA often are contaminated with hybrids: sense and antisense strands that form a double helix of double-stranded RNA (dsRNA). Double-stranded RNA corresponding to a particular gene is a powerful suppressant of that gene. In fact, the suppressive effect of antisense RNA probably also depends on its ability to form dsRNA (using the corresponding mRNA as a template).
The ability of dsRNA to suppress the expression of a gene corresponding to its own sequence is called RNA interference (RNAi). It is also called post-transcriptional gene silencing or PTGS.
Mechanism of RNAi
The only RNA molecules normally found in the cytoplasm of a cell are molecules of single-stranded RNA. If the cell finds molecules of double-stranded RNA (dsRNA), it uses an enzyme called Dicer to cut them into fragments containing ~21 base pairs (~2 turns of a double helix). The two strands of each fragment then separate — releasing the antisense strand. With the aid of a protein, it binds to a complementary sense sequence on a molecule of mRNA. If the base-pairing is exact, the mRNA is destroyed. Because of their action, these fragments of RNA have been named "small (or short) interfering RNA" (siRNA). The complex of siRNA and protein is called the "RNA-induced silencing complex" (RISC).
siRNAs can also interfere with transcription
There is growing evidence that siRNAs can also inhibit the transcription of genes
- perhaps by binding to complementary sequences on DNA or
- perhaps by binding to the nascent RNA transcript as it is being formed.
In fission yeast, at least, the siRNA is complexed with one molecule of each of three different proteins. The entire complex is called the RITS complex ("RNA-induced initiation of transcriptional gene silencing")
How these siRNAs — synthesized in the cytosol — gain access to the DNA in the nucleus is unknown.
Synthetic siRNA molecules that bind to gene promoters can — in the laboratory — repress transcription of that gene. The repression is mediated by methylation of the DNA in the promoter and, perhaps, methylation of histones in the vicinity.
There is a strain of rice (LGC-1) that produces abnormally low levels of proteins called glutelins. It turns out that of several glutelin genes found in rice
- two closely-similar glutelin genes are located back to back on the same chromosome.
- In LGC-1, a deletion has occurred between the two genes which removes the signal that would normally stop transcription after the first gene.
- Thus RNA polymerase II transcribes right past the first gene and on into the second.
- The result is a messenger RNA with almost-identical sequences running in opposite directions.
- This causes the mRNA to fold up into a molecule of double-stranded RNA (dsRNA).
- A Dicer-like enzyme cuts up the dsRNA into small interfering RNAs (siRNAs) that suppress further transcription of those genes as well as other glutelin genes.
Why RNAi?
RNAi has been found to operate in such diverse organisms as plants, fungi, and animals such as Drosophila melanogaster, Caenorhabditis elegans, and even mice and the zebrafish. Such a universal cell response must have an important function. What could it be?
Some possibilities:
- Some viruses of both plants and animals have a genome of dsRNA. And many other viruses of both plants and animals have an RNA genome that in the host cell is briefly converted into dsRNA. So RNAi may be a weapon to counter infections by these viruses by destroying their mRNAs and thus blocking the synthesis of essential viral proteins.
- Transposons may be transcribed into RNA molecules with regions that are double-stranded. RNAi could then destroy these.
- RNA interference may be the unexpected dividend of another basic process of controlling gene expression.
RNAi as a tool
In any case, the discovery of RNAi adds a promising tool to the toolbox of molecular biologists. Introducing dsRNA corresponding to a particular gene will knock out the cell's own expression of that gene. (Feeding C. elegans on E. coli manufacturing the dsRNA will even do the trick.)
Heroic Example
In the 24 March 2005 issue of Nature, Sönnichsen et al reported that they have injected dsRNAs corresponding to 20,326 of C. elegans's genes (98% of the total!) and monitored the effect of each on embryonic development from the completion of meiosis (following fertilization) through the second mitotic division that produces the 4-cell embryo.
They found that at least 661 different genes altered some process during this period:
- about half of them involved in cell division and
- half in general cell metabolism.
(Another thousand genes produced phenotypic effects that were seen at later stages of development.)
Because RNAi can be done in particular tissues at a chosen time, it often provides an advantage over conventional gene "knockouts" where the missing gene is carried in the germline and thus whose absence may kill the embryo before it can be studied.
Another Example: screening genes for their effect on drug sensitivity
- Distribute your cells in thousands of wells and add — from a "library" of thousands of siRNAs representing the entire genome — siRNA molecules targeting the expression of one gene to each well
- Add the drug to all the wells
- See which wells have cells that respond
Some other promising applications of RNAi
In mammalian cells
In mammalian cells, introducing dsRNA fragments only reduces gene expression temporarily. However, mammalian cells can be infected with a DNA vector that encodes an RNA molecule of 50–80 nucleotides called a "small hairpin RNA" (shRNA) containing a sequence corresponding to the gene that one wishes to suppress. As the shRNA is synthesized, dicer converts it into a typical siRNA molecule. Because the cell can continuously synthesize shRNA, the interference is long-lasting. In fact, with vectors that become integrated in the host genome, RNAi can be passed on to the descendants.
In plants
The 19 June 2003 issue of Nature reported on coffee plants that were engineered to express a transgene that makes siRNA that interferes — by RNAi — with the expression of a gene needed to make caffeine. So perhaps "decaf" coffee will one day no longer require the chemical removal of caffeine from coffee beans.
Monsanto is developing a transgenic corn (maize) that expresses a dsRNA corresponding to the sequence of an essential gene in the western corn rootworm, a devastating pest of the crop. After ingesting this dsRNA, the insect's own cells process it into an siRNA that targets the gene's mRNA for destruction and kills the worm in a few days.
Amplification of RNAi
In C. elegans, plants, and Neurospora, the introduction of a few molecules of dsRNA has a potent and long-lasting effect. In plants, the gene silencing spreads to adjacent cells (through plasmodesmata) and even to other parts of the plant (through the phloem). RNAi within a cell can continue after mitosis in the progeny of that cell. Triggering of RNAi in C. elegans can even pass through the germline into its descendants.
Such amplification of an initial trigger signal suggests a catalytic effect. It turns out that these organisms have RNA-dependent RNA polymerases (RdRPs) that uses the mRNA targeted by the initial antisense siRNA as a template for the synthesis of more siRNAs. Synthesis of these "secondary" siRNAs even occurs in adjacent regions of the mRNA. So not only can these secondary siRNAs target additional areas of the original mRNA, but they are potentially able to silence mRNAs of other genes that may carry the same sequence of nucleotides.
This phenomenon, called "transitive RNAi",
- may complicate the interpretation of gene suppression experiments as the expression of other genes may be suppressed in addition to the target gene;
- raises a warning flag for the use of RNAi to suppress single genes in human therapy (although RdRPs and amplification have not been observed in mammalian cells).
RNAi in human therapy
Because its target is so specific, the possibility of using RNAi to shut down the expression of a single gene has created great excitement that a new class of therapeutic agents is on the horizon. Many clinical trials are underway exploring the use of siRNA molecules in the treatment of a wide variety of diseases. To date, the most promising results have been using RNAi to target an inherited disease in which the liver secretes a mutant form of transthyretin leading to the accumulation of amyloid deposits in neurons and elsewhere.
MicroRNAs (miRNAs)
In C. elegans, successful development through its larval stages and on to the adult requires the presence of at least two "microRNAs" ("miRNAs") — single-stranded RNA molecules containing about 22 nucleotides and thus about the same size as siRNAs.
These small single-stranded transcripts are generated by the cleavage of larger precursors using the C. elegans version of Dicer.
They act by either destroying or inhibiting translation of several messenger RNAs in the worm (usually by binding to a region of complementary sequence in the 3' untranslated region [3'-UTR] of the mRNA).
The microRNAs (miRNAs) in C. elegans (which were first called "small temporal RNAs") turn out to be representatives of a large class of RNAs that are encoded by the organism's own genes.
- The initial product of gene transcription is a large molecule called pri-miRNA.
- While still within the nucleus an enzyme called Drosher cuts the pri-miRNA into a shorter molecule (~70 nucleotides) called pre-miRNA.
- The pre-miRNA is exported into the cytosol where it is cleaved (by Dicer in animals) into the miRNA.
MicroRNAs
- are found in all animals (humans generate some 1000 miRNAs) and plants but not in fungi.
- contain 19–25 nucleotides;
- are encoded in the genome
- some by stand-alone genes (that may encode several miRNAs)
- some by portions of an intron of the gene whose mRNA they will regulate.
- may be expressed in
- only certain cell types and
- at only certain times in the differentiation of a particular cell type.
While direct evidence of the function of many of these newly-discovered gene products remains to be discovered, they regulate gene expression by regulating messenger RNA (mRNA), either
- destroying the mRNA when the sequences match exactly (the usual situation in plants) or
- repressing its translation when the sequences are only a partial match. In this latter case, it probably requires several miRNAs to bind simultaneously in the 3'-UTR.
MicroRNAs have two traits ideally suited for this:
- Being so small, they can be rapidly transcribed from their genes.
- They do not need to be translated into a protein product to act (in contrast, e.g., to transcription factors).
MicroRNAs regulate (repress) expression of genes in mammals as well. Genome analysis has revealed thousands of human genes whose transcripts (mRNAs) contain sequences to which one or more of our miRNAs might bind. Probably each miRNA can bind to as many as 200 different mRNA targets while each mRNA has binding sites for multiple miRNAs. Such a system provides many opportunities for coordinated mRNA translation.
A study reported in Nature (Lim, et al., 433: 769, 17 Feb 2005) used DNA chip analysis to show that when a particular miRNA was expressed in HeLa cells,
- a miRNA normally expressed in the brain repressed mRNA production by 174 different genes while
- a miRNA normally expressed in cardiac and skeletal muscle repressed mRNA production by 96 genes — all but 8 of them different from the those repressed by the brain miRNA.
As work proceeds rapidly in this field, the pattern that begins to emerge is that:
- Many genes — especially those involved in such housekeeping activities (e.g., cellular respiration) common to all cells — do not have 3'-UTRs that can be blocked by any of the miRNAs encoded in the genome.
- The genes that must be expressed in a particular type of differentiated cell and/or at a particular time in the life of that cell
- do not express any of the miRNA genes that could block their expression but
- do express miRNA genes that block the expression of other genes for specialized functions that would not be appropriate in that cell at that time.
- Rather than being simple switches that turn gene expression on or off, miRNAs seem to exert a more subtle effect — raising or lowering the level of gene expression (much as protein transcription factors do).
Thus repression of gene expression by miRNAs appears to be a mechanism to ensure regulated and coordinated gene expression as cells differentiate along particular paths. For example, when zygote genes begin to be turned on in the zebrafish blastula, one of them encodes a miRNA that triggers the destruction of the maternal mRNAs that have been running things up to then.
So miRNAs may play as important role as transcription factors in regulating and coordinating the expression of multiple genes in a particular type of cell at particular times.
Therapeutic miRNAs?
The ease with which miRNAs can be introduced into cells and their widespread effects on gene expression have given rise to hopes that they might be useful in controlling genetic disorders, e.g., cancer.
To date, some laboratory studies have been quite promising.
- A miRNA that blocks the expression of G1 and S-phase cyclins — and thus stops the cell cycle in its tracks — protects mice from liver cancer.
- a miRNA that inhibits genes needed for metastasis suppresses the metastasis of treated human breast cancer cells.
Summary
In addition to protein transcription factors, eukaryotes use small RNA molecules to regulate gene expression — almost always by repressing it — so the phenomenon is called RNA silencing.
There are two sources of small RNA molecules:
- small interfering RNAs (siRNAs)
- Plant cells make these from the double-stranded RNA (dsRNA) of invading viruses.
- Scientists and pharmaceutical companies make these as agents to turn off the expression of specific genes (called RNA interference or RNAi).
- micro RNAs (miRNAs)
- These are encoded in the genomes of all plants and animals.
- Both siRNAs and miRNAs are processed in the same way in the cytosol of the cell.
- Both are generated by Dicer.
- Both are incorporated into an RNA-induced silencing complex (RISC).
- If the nucleotide sequence of the small RNA exactly matches that of the mRNA, the mRNA is cut and destroyed.
- If there is only a partial match (usually in its 3' UTR), translation (i.e., protein synthesis) is repressed. Both of these activities take place in the cytosol — perhaps in P bodies.
- However, for some small RNAs, the RISC complex enters the nucleus and turns off transcription of the corresponding gene(s) by
- binding to the unwound DNA sequence (or perhaps the RNA transcript as it is being formed)
- converting euchromatin to heterochromation
- methylating of lysine-9 histone H3 in the nucleosomes around the gene(s)
Aside from their use as laboratory — and perhaps therapeutic — tools, small RNAs are clearly essential to the organisms that make them.
Some examples:
- Plants and animals use them to defend themselves against viruses.
- Example: When human cells are infected by hepatitis C virus (HCV), they produce miRNAs that interfere with gene expression by this RNA virus and thus its ability to replicate.
- Some herpesviruses use them to keep their host cell alive long enough to complete viral replication (by blunting a host immune response against the infected cell and preventing its premature death by apoptosis).
- Of the 46 miRNAs expressed in the Drosophila embryo, 25 have been shown to be essential to normal development.
- Correct embryonic development in other animals (e.g., C. elegans, zebrafish, mice) also requires them.
- They protect against the danger of mutations caused by transposons moving around in the genome.
- They are also needed to regulate the size of the pool of at least some types of stem cells.
- Transgenic mice with a single miRNA gene knocked out develop severe immunodeficiency affecting dendritic cells, helper T cells, and B cells.
- Reduced, or no, expression of certain miRNAs are characteristic of several different cancers in humans.


