1.9: Biomolecule Detection
- Page ID
- 36751
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
Goals:
- Employ indicators to discover characteristics of a solution.
- Use indicators to determine contents of an unknown solution.
- Employing positive and negative controls to validate a test.
Student Learning Outcomes:
Upon completion of this lab, students will be able to:
- Describe the properties of some important biomolecules.
- Explain important characteristics of proteins and carbohydrates.
- Perform tests to detect the presence of carbohydrates and proteins.
- Explain the importance of a control in biochemical tests.
- Use a biochemical test to identify the presence of a molecule in an unknown solution.
INTRODUCTION
The Macromolecules of Life: Proteins, Carbohydrates, and Lipids
The cells of living organisms are composed of large molecules (macromolecules) sometimes also referred to as organic molecules because of the presence of the element carbon. Very many of the organic molecules found in living organisms are carbohydrates, proteins, lipids, and nucleic acids. Each of these macromolecules is made of smaller subunits. The different molecules have different chemical properties. For example, monosaccharides such as glucose will react with a chemical agent called Benedict’s solution but disaccharides, like sucrose, and polysaccharides, like starch will not. Similarly, proteins will react with a mix of potassium hydroxide and copper sulfate but free amino acids, carbohydrates, and lipids will not.
Today, we will focus on three of these molecular types: lipids, proteins and carbohydrates. You will work with nucleic acids in another lab. You may want to review the properties of the biomolecules of life.
| Molecular Type |
Molecular Structure |
Macro Structure |
|---|---|---|
|
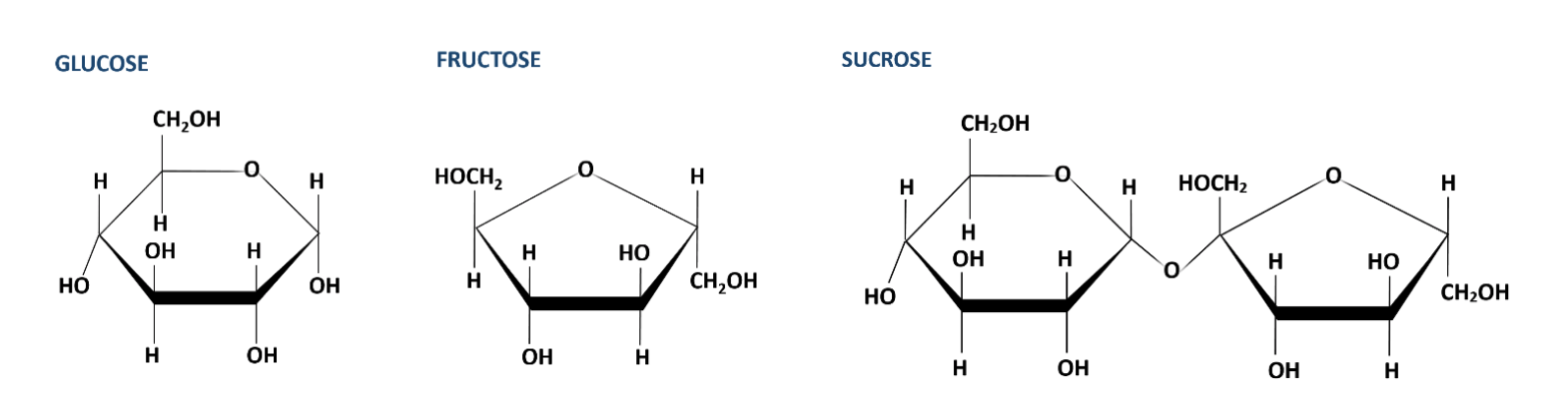
Sucrose |
|
Common source: Table sugar |
|
Starch |
|
Common source: Rice |
|
Lipids |
|
Common source: Cooking oils |
|
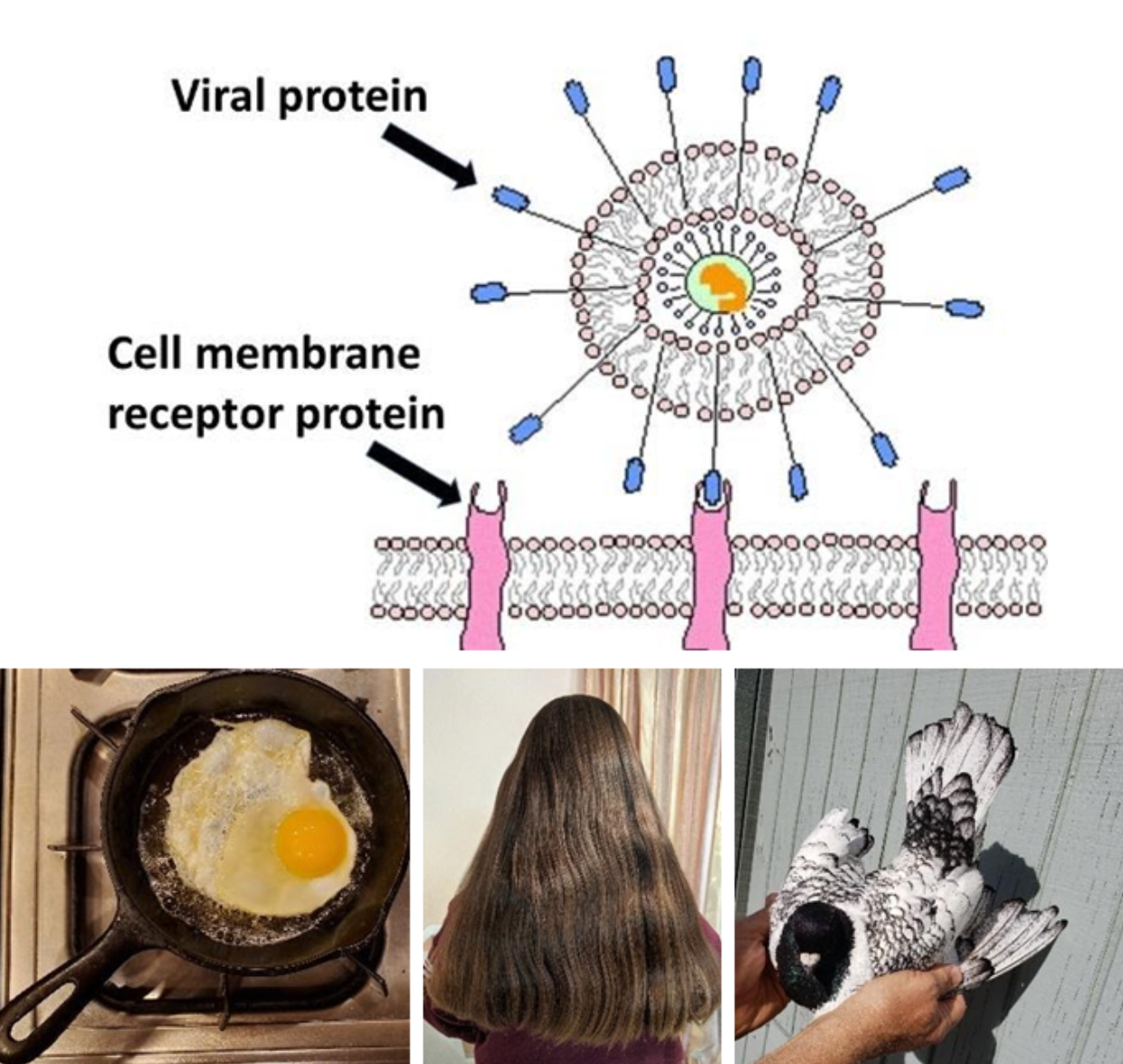
Proteins |
|
Common sources: cell receptors, egg, hair, feathers
|
Part I: Controlled Experiments to Identify Organic Compounds
Indicators are chemicals that change color when chemical conditions change, such as pH, or when a chemical reaction takes place producing a colored molecule. There are many biochemical procedures that can be used to detect the presence of important molecules. In this exercise, you will test various solutions in order to detect the presence of these molecules. We will employ controls as we test the solutions. Controls provide results to compare to the solution being tested. Controls should give predictable results. By comparing the test solution result with the controls, you can determine the result of the test solution.
A positive control contains the variable for which you are testing. When the positive control is tested, it reacts in an expected manner. If, for example, you are testing for a type of carbohydrate in unknown solutions, then an appropriate positive control is a solution known to contain that type of carbohydrate. The resulting reaction, when properly performed, will demonstrate that the reagents work as expected and shows what the result should look like if the test solution is positive. If the positive control does not react as expected, your test is not valid. Perhaps your test reagents are not working properly.
A negative control does not contain the variable for which you are testing. Often a negative control contains only water. It will not react with the indicator reagents. Like the positive control, the negative control solution shows you what a negative result looks like and verifies that the detecting reagent is working properly. If the negative control does react, your test result is not valid. Perhaps the control solution or reaction tube was contaminated with the test variable.
I. Carbohydrates
Benedict’s Test for Monosaccharides
Molecules made of the atoms carbon (C), hydrogen (H), and oxygen (O), in a ratio of 1:2:1 are carbohydrates. For example, glucose, one of the most important carbohydrates for living cells, has the chemical formula C6H12O6. Simple sugars also known as monosaccharides are carbohydrates. Paired monosaccharides form disaccharides. A common example of a disaccharide is the table sugar, sucrose. It is composed of the monosaccharides glucose and fructose linked to fructose. Similarly, linking three or more monosaccharides forms a polysaccharide. Starch, glycogen, or cellulose are polysaccharides important to cells and have many monomers of glucose linked together in different ways.
Benedict’s reagent is the indicator we use to detect monosaccharides. When monosaccharides are mixed with Benedict’s and heated, a color change occurs. If there is a small amount of monosaccharide in the solutions, a greenish solution is produced. If the solution contains a large amount of monosaccharide, an orangish precipitate results. A precipitating solution means small particles settle out of the solution.
Monosaccharides + Benedict’s reagent + Heat ⇒ Green to Orange
II. Proteins
The cell relies on proteins for very many functional reasons. Proteins may be enzyme catalysts, form channels for molecules to pass across membranes, form structures and more. The subunit of protein molecules are monomers of amino acids. The bond that forms between amino acids to form protein is called a peptide bond.
Peptide bonds can be detected by using two chemical reagents, potassium hydroxide (KOH) and copper sulfate (CuSO4). Potassium hydroxide causes a protein to break apart so that copper sulfate can react with the peptide bonds. The resulting color is purple. The more protein, and hence more peptide bonds, in the solution, the darker the resulting purple will become.
Testing for Monosaccharides with Benedict’s Reagent
Proteins + KOH + CuSO4 ⇒ Purple
Materials
- Test tubes labeled with the contents you will add to each tube
- Beaker with water and hot plate (water heated to near boiling)
- Metric ruler
- Marker
- Deionized water and carbohydrate solutions
- Appropriate tool to remove hot tubes from water
Procedure
- Obtain 5 test tubes and number them 1 – 5.
- Use a marker to indicate 2.5 cm from the bottom and another mark at 5cm from the bottom.
- Fill each test tube to your 2.5 cm mark with the appropriate solution:
1. Distilled water 2. Concentrated glucose solution 3. Diluted glucose solution 4. Sucrose solution 5. Starch solution - Add Benedict’s solution to each tube to the 5 cm mark.
- Place all of the tubes in a hot (90°C) water-bath for 2 min, and observe color-changes during this time.
- After 2 min, remove the tubes from the water-bath and record the color of their contents in the table below. Also observe your classmate’s reactions.
Observations
Perform the Benedict’s test for monosaccharides. Reproduce this table in your lab book and complete it with your observations.
|
Tube Contents |
Color after reaction |
Presence of monosaccharide? |
|---|---|---|
|
1. Water |
||
|
2. Concentrated glucose |
||
|
3. Diluted glucose |
||
|
4. Sucrose solution |
||
|
5. Starch solution |
Instructions to clean up
* Clean tubes are very important. Contaminated tubes may influence results of future tests.
1. When your observations are complete, carefully wash and rinse the tubes following the instructions in part 2. You may leave the markings on them until the final clean up procedure of the day.
Data Analysis
- Which of the above solutions serve as your positive control? Negative control?
- Examine your test and your classmates test solutions. Which solutions were positive for monosaccharides?
- Which contains a higher concentration of monosaccharides, potato juice or onion juice? How do you know?
- Which solutions did not react with the Benedict’s solution?
Testing for Peptide Bonds (Protein)
Materials
- Four clean test tubes labeled with the contents you will add to each tube
- deionized water, and test solutions
- Indicator reagents potassium hydroxide (KOH) and copper sulfate (CuSO4)
Procedure
Perform the Peptide Bond test for Protein
 Do not spill the KOH – it is extremely caustic. Rinse your skin if it comes in contact with KOH.
Do not spill the KOH – it is extremely caustic. Rinse your skin if it comes in contact with KOH.
- Use your four clean test tubes from the previous procedure. They still need to be numbered and marked at 2.5 and 5 cm from the bottom.
- Fill each test tube to the 2.5 cm mark with the appropriate solutions indicated below
- Water
- Protein Solution
- Amino Acid Solution
- Test Solution
- Add potassium hydroxide (KOH) to the 5cm mark on each test tube.
- Add five drops of copper sulfate (CuSO4) to tube and mix well.
- Record the color of the tubes’ contents in the table below. Also observe your classmate’s reactions.
- When finished dump the contents of the tubes and wash them. Rinse with distilled water.
Observations
Perform the Protein Test: Reproduce this table in your lab book and complete it with your observations.
|
Tube Contents |
Color after reaction |
Presence of protein? |
|---|---|---|
|
Water |
||
|
Protein solution |
||
|
Amino acid solution |
||
|
Unknown solution |
Instructions to clean up
*Clean tubes are very important. Contaminated tubes may influence results of future tests.
When your observations are complete, carefully wash and rinse the tubes following the instructions in Part I.
Data Analysis
- Which of the solutions is a positive control? Which is a negative control?
- Do individual amino acids have peptide bonds? How do you know this to be true?
- What type of solution did you test as your unknown? Did it contain protein?
- Observe your classmates reactions and describe which unknown solutions contain the most and the least protein. How can you tell?
III. Lipids
Lipids are a class of molecules that are not soluble (do not dissolve) in water. They are composed of the molecular building blocks of glycerol and three fatty acids. Fatty acids come in two major types, saturated and unsaturated. This difference is due to the presence of particular types of bonds within the fatty acid molecule (see figure) and affect the shape and characteristics of the overall lipid containing these fatty acids. You may want a review of lipids.
Testing for Lipid with Sudan IV
 Use gloves and avoid contact with Sudan IV as it is considered a possible carcinogen. Immediately wash your skin with soap and plenty of water if you come in contact with the solution.
Use gloves and avoid contact with Sudan IV as it is considered a possible carcinogen. Immediately wash your skin with soap and plenty of water if you come in contact with the solution.
Materials
- Filter paper (small enough to fit in the petri dish) and pencil with areas labeled for test substances
- clean empty petri dish
- solution of 0.2% Sudan IV
- Gloves (see safety warning)
- Dedicated transfer pipettes or micropipettes with tips.
- Solutions of deionized water, vegetable oil, and test solutions (cream, dairy milks, coconut milk, soy milk etc.)
- optional- hairdryer
Procedure
- Obtain filter paper and on the far edge mark with pencil which solutions will be placed toward the interior of the mark.
- Drop a small amount of solution near the appropriate mark. 1. Distilled water 2. Vegetable oil 3-6. Test solutions
- Allow to dry. Use a hairdryer to speed up this process.
- While the paper is drying, answer the Data Analysis questions below.
- Soak the paper in the petri dish containing 0.2% Sudan IV. (handle with gloved hands)
- Rinse the paper in distilled water and allow to dry.
- Record the color of the spots in the table below. Also observe your classmate’s reactions.
Observations
Sudan IV test for lipid: Reproduce this table in your lab book and complete it with your observations. The darker the stain, the more lipid is present.
|
Spot Contents |
Color after reaction |
Relative amount of lipid? |
|---|---|---|
|
1. Water |
||
|
2. Vegetable oil |
||
|
3. |
||
|
4. |
||
|
5. |
||
|
6. |
Instructions to clean up:
When your observations are complete, carefully dispose of any remaining Sudan IV solution in the container provided by your instructor. Always use gloves and do not move the container if there is a danger of spilling.
Data Analysis
- Which of the above solutions serve as your positive control? Your negative control?
- Hypothesize which solutions will contain the greatest amount of lipid. Why do you believe this to be true?
- Which solutions contained the greatest amount of lipid?
- Did your observations support your hypothesis? Were you surprised by some of the results? Explain.
Part II. The Saga of the Soda Dispenser
Enrique was a new employee. This was his first job and he had only been on the job for a couple of weeks and was still on “hiring probation.” He liked the crew he worked with and the paycheck that would come every few weeks. He wanted to stay. Today, there was a problem and he had to figure out something fast to solve it. He knew that if he did, the manager would be really pleased and his job was guaranteed.
Someone was complaining that the soda dispenser was dispensing “regular” cola from the “diet cola” dispenser. The customer claimed to be on a reduced-calorie diet and was not happy about the extra calories consumed. There was more at stake than one unhappy customer, though. The manager told Enrique that many of their customers were diabetic and consuming sugar-laden soda could alter their blood-sugar chemistry in a dangerous way. They could not allow those customers to be harmed.
Scope of the Problem
If the diet soda dispenser did have regular soda, then did the regular soda dispenser have diet? What about the Dr. Pepper dispenser? That, at least, tasted like Dr. Pepper, so it was OK- or was it? What a mess! Should they throw all the soda in the dispenser out and start again? Or was there some way of determining if the soda was being dispensed correctly? If they could determine what the problem was, they could save the business money and not waste the soda products.
Enrique’s Attempt to Solve the Mystery
Enrique knew that most soda had high fructose corn syrup in it but diet soda had sugar substitutes in it: Substitutes that were not sugar but fooled your taste buds into believing it was.
Questions for your lab book:
- Does the regular soda have high fructose corn syrup in it? Look at the label determine if it does or doesn’t. Write your observation in your lab book.
- Does the diet soda have high fructose corn syrup in it? Look at the label determine if it does or doesn’t. Write your observation in your lab book.
- Determine whether fructose is a monosaccharide, disaccharide or polysaccharide.
- Can we do a test?
Just the other day, in science lab, Enrique had run some tests on solutions in order to determine their compositions. One of the tests was for detecting monosaccharides in solution! He knew his science teacher would still be in the classroom at this time and the school was barely a 5 minute walk from the restaurant. He could solve the mystery in under 30 minutes! Enrique quickly told his manager his plan and grabbed some cups of soda, which he labeled, so he could tell which dispenser they came from, then headed out. Enrique quickly ran to the school lab and got permission to run his experiment. Help Enrique set up an experiment to test the soda.
More questions for your lab book:
- Would it be a good idea to include controls? If so, which solutions?
- Which detector reagent(s) will you use?
- What colors will you look for to indicate the presence of the “regular” soda?
- How many test tubes do you need? How will you label them?
Testing Unknown Soda Solutions
Materials
- Clean test tubes labeled with the contents you will add to each tube
- deionized water, and solutions to test
- Indicator
Procedure
Perform the test for monosaccharides:
- Obtain the needed number of clean test tubes and mark them at 2.5 and 5 cm as before. Code them as to the contents (numbers corresponding to your solutions- which you record below)
- Obtain the unknown solutions from your instructor.
- Fill the tubes to the 2.5 cm mark with the control and test substances.
- Fill the tubes to the 5 cm mark with indicator and treat was needed.
- Reproduce this table in your lab book and complete it with your observations, then answer the questions regarding the soda saga.
Observations
Perform the Appropriate Test: Reproduce this table in your lab book and complete it with your observations.
|
Tube Contents |
Color after reaction |
Presence of fructose? |
Diet or regular? |
|---|---|---|---|
|
1. |
|||
|
2. |
|||
|
3. |
|||
|
4. |
|||
|
5. |
|||
|
6. |
Instructions to clean up:
 DO NOT allow ethanol to come in contact with the hotplate. Ethanol is very flammable.
DO NOT allow ethanol to come in contact with the hotplate. Ethanol is very flammable.
*Clean tubes are very important. Contaminated tubes may influence results of future tests.
- When your observations are complete, carefully wash and rinse the tubes following the instructions in part 1.
- At the end of the lab period be sure all labels are removed from the tubes using a small piece of paper towel and ethanol.
Final Conclusion
- What does Enrique tell his manager? Is the soda dispenser messed up or not?
- What, if any, soda needs to be changed?
Study Questions
- Why should you always include controls in each procedure?
- What serves as a good negative control and why?
- Describe a positive control.
- If you run a test for monosaccharide on what you believe is “regular” lemon lime-flavored soda, but the solution is sky-blue after heating with Benedict’s what does this tell you?
- What if only AFTER running your test, you read the label of the lemon-lime soda and notice that the ingredients do not contain fructose but does contain sucrose. Is your test procedure faulty or is there another explanation for your result?
Attributions
- Sucrose Molecular Structure from LibreTexts 5.2 Carbohydrates.
- Protein Structure diagram by Lady of Hats, Public Domain, via Wikimedia Commons.
- Amino Acids forming a peptide bond (bottom image) by OpenLab at CitiTech CC-BY-NC-SA