1.4: The Scientific Method
- Page ID
- 36746
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
Goals:
- Utilize the steps in the scientific method to design, collect and interpret scientific data.
Student Learning Outcomes:
Upon completion of this lab, students will be able to:
- Formulate a hypothesis based on an observation.
- Design their own experimental method including proper controls.
- Collect results and describe colony growth (morphology) present.
- Determine if data collected supports their hypothesis
INTRODUCTION
Microbes are all around us. In this lab, you will be introduced to this as well as to the scientific method. Throughout the semester, you will learn how to aseptically work with bacteria; meaning learning how to culture bacteria without contaminating yourself and keeping a pure sample of bacteria free from other unwanted bacteria. During this lab exercise, you are going to swab an area of your choice to see what bacteria and/or fungi are present there. You will also test the effect of some disinfectants on the bacterium Escherichia coli (E. coli) Using the scientific method, you will design an experiment, collect data, and interpret your results.
The scientific method is a generalized tool used to aid in asking and answering a scientific question by making observations and performing experiments. There are steps that are generally followed when conducting and designing an experiment. First, an initial observation is made. An observation can involve noting any event (a pattern, an action, a behavior, or a reaction). After making an observation, a question can be asked about the event. Once a question is asked, then research regarding what is already known relating to this question (finding background material) can be discovered to better understand the observation. This background information typically comes from publications in scientific literature, such as journal articles and reviews. Once the background information is understood, a hypothesis can be formed. This gathering of information and its application to a solution is an example of inductive reasoning. The hypothesis is then either supported or rejected depending on the analysis of the results of well-designed experiments. Each experiment needs dependent and independent variables. The value of the dependent variable is determined and is a function of the independent variable. In an ideal experimental setup, the independent variable is something over which we have some control and changes in some predetermined way, while changes in the dependent variable are observed and measured. A hypothesis must include both of these variables. A hypothesis can be generated by creating an “if-then” statement. For example, “If I treat cancer cells with drug x then they will die. “
Part I: Disk Diffusion Method to Evaluate Disinfectants
For this portion of the lab, you will be provided the protocol/instructions but you will choose the substances to test. You will develop and test your hypothesis.
Materials
- 1 culture of E. coli
- sterile swabs (1 swab needed per plate)
- sterile absorbent paper disks
- sterile water (negative control)
- 10% bleach (positive control)
- 30% hydrogen peroxide (positive control)
- 4 test disinfectant solutions
- 1 petri plate (containing sterile nutrient agar)
Method
- Plan your experiment. In addition to the controls, which solutions would you like to test?
- Answer parts A, B, and C below to help with your planning.
- Dip the sterile swab into the E. coli solution then spread it over the entire surface of the NA plate by rubbing the swab over the entire surface. You want to coat the entire surface with the bacteria so do not leave spaces that have not been in contact with the swab. Be careful to only open the lid of the plate enough to work (like a clamshell). If you open the lid all the way, you risk contaminating the surface with unwanted bacteria/fungi from the environment.
- Dispose of the swab in the appropriate waste container.
- Using sterile forceps (tweezers) dedicated to the solution to be tested, dip sterile disks one at a time into the following solutions and place them onto the agar surface that has been inoculated with E. coli. Be sure not to allow the tweezers themselves to come in contact with the agar because that will cause them to become contaminated with bacteria.
- Include all answers to your questions in your lab notebook along with your procedure for testing the disinfectants.
Observation
- Based on your experience and observations, which solutions do you think will inhibit the growth (or kill) E. coli the most? Which solutions are you interested in testing?
Hypothesis
- Based on your observations, write the hypothesis you wish to test.
Experimental Design
Work with your group to write a protocol for your experiment based on the questions below. Start with the instructions and insert the necessary details such that a person with no knowledge of your project would be able to read your protocol and fully understand what to do.
- Based on your hypothesis, which solutions will have the largest zone of inhibition around the disks?
- What will you include as your experimental controls? (Which solutions WILL or will NOT inhibit the bacteria)?
- How will you set up your experiment? (I recommend writing a map on your plate on the agar side where you will place the disks and then making a key to the map in your lab notebook).
Example Protocol
- On the bottom of your NA agar plate (the side with the agar, NOT the lid!!), label the plate using a permanent marker with your initials, “Biotech Lab”, the date, E. coli test, and where you are placing each disk.
- Take the sterile swab and dip it in the E. coli culture. (Don’t place the lid for the E. coli on the desk or it will now be contaminated!) Place the labeled plate on the desk in front of you with the lid side of the plate up. With one hand, open the lid (only open it a little bit so that you can have access to the agar; think of a clam shell) and use the swab to spread the bacteria all over the plate. Make sure to move the swab around to cover the entire plate.
- Cover the plate with the lid and discard the swab in the appropriate waste container
- Using dedicated forceps, dip a sterile paper disk into a solution to be tested and then place the disk onto the E.coli-inoculated surface. Open the plate like a clamshell each time you place a disk then close the lid immediately when finished.
Figure 2. Zone of inhibition - Place plate in a 37⁰C incubator, with the agar side up. (note: you can place it into the same 32⁰C incubator as the next experiment)
- The plate will grow in the incubator for 48 hours.
- When incubation is complete, measure the diameter of any zones of inhibition using a millimeter (mm) scale. Report data in the table below.
Results
- Remove your plates from the incubator. DO NOT OPEN THE PLATES!
- Take a picture of your plates and include them in your lab notebook. Be sure to clearly label each portion of the plate.
- Make the following table in your notebook and record your data.
Solution Tested |
Diameter of Inhibition Zone (mm) |
|---|---|
Conclusion
- Which solution did you use as a negative control? Did this control provide the expected result?
- Based on your observations, which solution had the greatest effect on the E. coli? Which has little or no effect?
Part II: Environmental Sampling
For this portion of the lab, you will develop and test your hypothesis as well as design the method to test it.
Materials
- 1 tube of sterile water
- sterile swabs (1 swab for each sample to be collected)
- Luria broth (LB) or nutrient agar (NA) plates (1 plate for each sample to be collected)
Method
- Working with your group, determine your experimental design for this lab.
- Complete parts A, B, and C below to help with your planning.
- Include all answers to your questions in your lab notebook along with your procedure for collecting your samples.
Observation
- Based on your observations of the world around you what surfaces do you think are most “dirty” or “clean”? Which surfaces are you interested in testing?
Hypothesis
- Based on your observations write the hypothesis you wish to test in your lab notebook.
Experimental Design
- Based on your hypothesis, how many surfaces/samples will you test?
- What will you include as your experimental control?
- How will you perform your experiment? (I recommend dipping your sterile swab into the sterile water and then swabbing your sample).
- How long and in which pattern will you swab your samples? (roll, zigzag, etc.)
- How many plates will you need and how will you section them? (you can use a sharpie to label the bottom of the plate and draw sections if needed).
Based on these questions: Work with your group to write a protocol for your experiment. Include enough detail that a person with no knowledge of your project would be able to read your protocol and fully understand what to do. Below is a general protocol for this lab to help you get started.
Example Protocol
- On the bottom of your NA agar plate (the side with the agar, NOT the lid!!), label the plate using a permanent marker with your initials, “Biotech Lab”, the date, and where you are choosing to swab.
- Take the sterile swab and dip it in the sterile water (don’t place the lid for the sterile water on the desk or it will now be contaminated!). Then touch the wet swab to whatever surface you would like to test in order to pick up the bacteria. Place the labeled plate on the desk in front of you with the lid side up. With one hand, open the lid (only open it a little bit so that you can have access to the agar; think of a clam shell) and use the swab to spread the bacteria all over the plate. Make sure to move the swab around to cover the entire plate.
- Cover the plate with the lid and discard the swab.
- Place plate in a 32⁰C incubator, with the agar side up. (Some organisms in the environment do not grow well at 37⁰C.)
- The plate will grow in the incubator for 48 hours.
Results
- Remove your plates from the incubator. DO NOT OPEN THE PLATES!
- Take a picture of your plates and include them in your lab notebook. Be sure to clearly label each portion of the plate.
- Make tables 2 and 3 in your notebook; record your data.
Sample Collected |
Number of Colonies Present |
|---|---|
Sample Collected |
Morphology of Colonies Present |
|---|---|
Use the image below to help you describe the morphology of the colonies present on your plates.
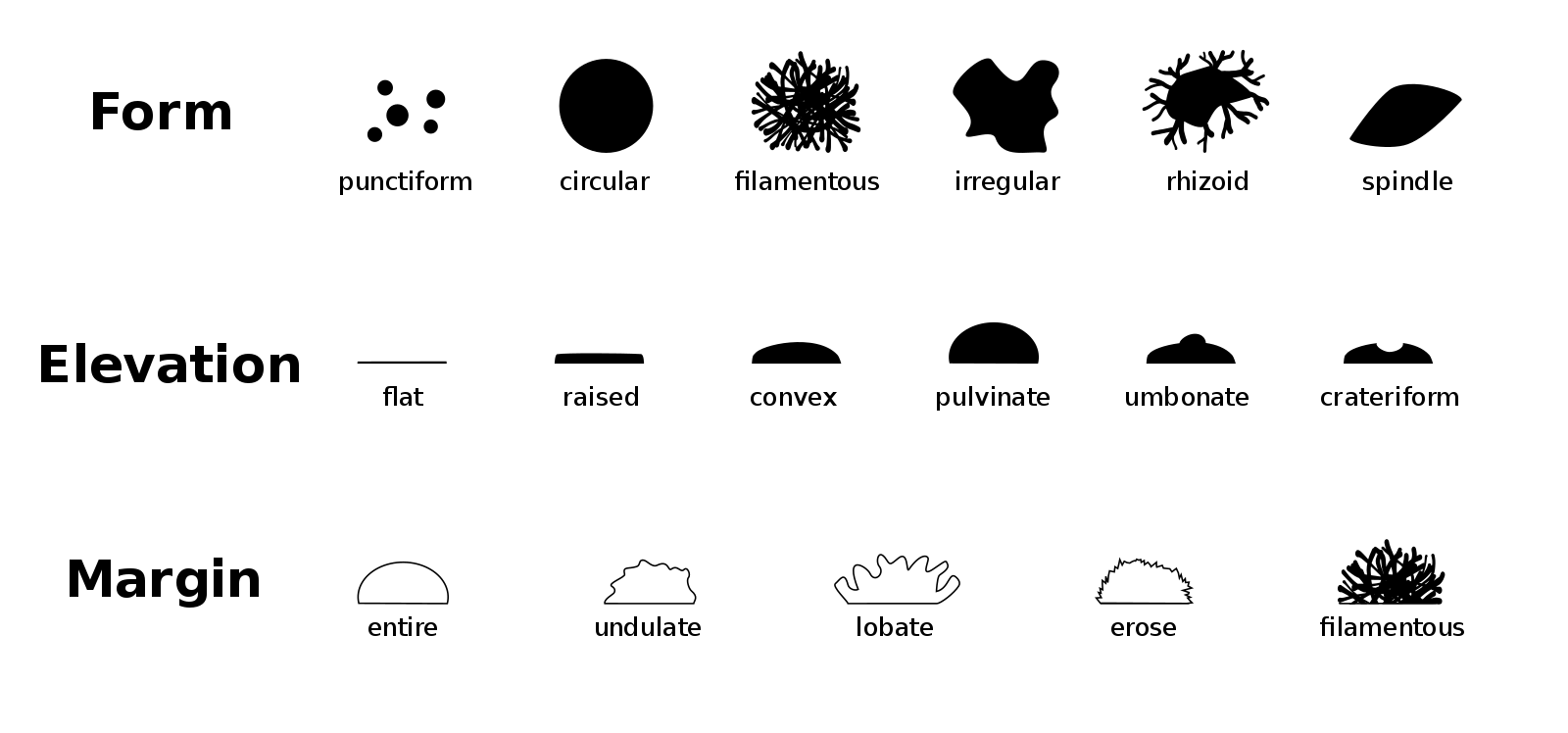
Conclusion
- Based on your experimental data, which surfaces had the most bacterial/fungal growth?
- Which surface had the most diverse number of bacteria/fungi?
- Based on your observations, what types of bacteria/fungus do you think were present on your plate?
Study Questions
- What are the steps of the scientific method?
- Be able to write a hypothesis based on a given observation.
- What is the purpose of an experimental control?
- What is the definition of an independent variable? A dependent variable?
- Why do we incubate plates upside down?
- Why do we label the agar side of the plate?
- What is the purpose of incubating the plates?
- What is the purpose of the LB or NA in the plate?
- Given a set of data, be able to formulate a conclusion based on the results given.


