41: Nitrate Reduction
- Page ID
- 3690
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Identify the different ways that nitrate can be reduced by bacteria
NITRATE TEST
This test determines the production of an enzyme called nitrate reductase, resulting in the reduction of nitrate (NO3): the test is also called the nitrate reduction test. With this enzyme, nitrate is reduced to nitrite (NO2). It then forms nitrous acid that reacts with the first reagent sulfanilic acid, and that reacts with the other reagent naphthylamine to form a red color.
Reduction of nitrate is generally an anaerobic respiration in which an organism derives its oxygen from nitrate.
MATERIALS NEEDED
- 1 nitrate broth for the unknown
- AFTER INCUBATION:
- nitrate reagents A (sulfanilic acid) and B (naphthylamine)
- wooden sticks for zinc
- zinc powder
THE PROCEDURE
 Inoculate the nitrate broths with your bacterial unknown.
Inoculate the nitrate broths with your bacterial unknown.- Incubate at the optimal temperature, 30º C or 37º C, for your organisms.
- AFTER INCUBATION: Look for N2 gas first before adding reagents.
- Add 6-8 drops of nitrite reagent A.
- Add the same number of drops of nitrite reagent B.
- You should see a reaction within a minute or less.
- If you have not seen either nitrite or N2 gas, you need to add a bit of powdered zinc.
- A bit of zinc is about the amount that sticks to the end of a wood stick.
- The reduction of unused nitrate by zinc takes a couple of minutes.
INTERPRETATION
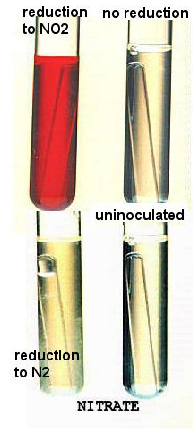
There are various ways that a bacterium can utilize nitrate as the final electron acceptor in anaerobic respiration. The first obvious product of reduction to look for is reduction to N2 gas, called denitrification, within the Durhm tube. This is looked for FIRST before any reagents are added.
If there is no nitrogen gas, there are still a couple of possible interpretations---nitrate reduction to nitrite (NO2), reduction to ammonia, or no reduction of nitrate at all.
A red color will be produced in the medium only when nitrite is present in the medium.
There may be 2 explanations for the lack of nitrite:
- The nitrate may not have been reduced; the bacterium cannot use nitrate (a – test)
- The nitrate may have been reduced to nitrite which has then been completely reduced to ammonia.
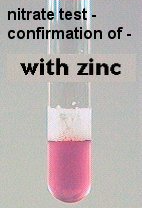
TO DIFFERENTIATE BETWEEN THE ABOVE 2 POSSIBILITIES, powdered zinc is added. If the bacterium has not used the NO3, it will still be present in the tube. The Zn will reduce the nitrate, forming nitrite, which then reacts with the 2 reagents ALREADY added to the tube. A pink-red color will form as confirmation of a NEGATIVE nitrate reduction.
The last possibility, production of ammonia, is indicated when no pink forms.
| REACTION | N2 gas | Color after adding reagents | Color after adding zinc |
| NO3 to NO2 | none | red | (Zn not added) |
| NO3 to N2 | yes | no color | (Zn not added) |
| NO3 to ammonia | none | no color | no color |
| NO3 - no reaction | none | no color | pink-red |
QUESTIONS
- Name the 2 major end products of nitrate reduction.
- WHY add zinc powder? WHEN do you add it?
- How do the definitions of nitrate reduction and denitrification differ?
- Is nitrate reduction an aerobic pathway or an anaerobic pathway?
Contributors and Attributions
-
Jackie Reynolds, Professor of Biology (Richland College)

