1: Media Preparation
- Page ID
- 3250
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Understand how to make media, how to sterilize it, and how to distribute it in different formats.
- Produce TSA plates, TSA slants, and TSB which will be used in subsequent lab periods.
- Understand the basics of an autoclave and how it sterilizes, including parameters.
Bacteria and fungi are grown on or in microbiological media of various types. The medium that is used to culture the microorganism depends on the microorganism that one is trying to isolate or identify. Different nutrients may be added to the medium, making it higher in protein or in sugar. Various pH indicators are often added for differentiation of microbes based on their biochemical reactions: the indicators may turn one color when slightly acidic, another color when slightly basic. Other added ingredients may be growth factors, \(\ce{NaCl}\), and pH buffers which keep the medium from straying too far from neutral as the microbes metabolize.
In this exercise, you will make all-purpose media called trypticase soy broth and trypticase soy agar. These 2 media----one a liquid and the other a solid---are the exact same formula save for the addition of agar agar (really- agar agar), an extract from the cell walls of red algae.
The old way to make media was by the cookbook method--- adding every ingredient bit by bit. The only time that is done today is when making a special medium to grow a certain finicky organism, where particular growth factors, nutrients, vitamins, and so on, have to be added in certain amounts. This medium is called a chemically defined medium (synthetic). Fortunately, the most common bacteria that we want to grow will do nicely with media that we commonly use in lab. Some of our media is bought, but most is produced in the prep area behind the lab. Since this type of medium has some unknown ingredients, or sometimes unknown quantities it is called complex media.
It is really very simple to make complex media these days:
- rehydrate the powder form of the medium
- stir and boil the agar medium to get the agar powder dissolved (if making an agar medium rather than a broth medium)
- distribute the medium into tubes
- autoclave to sterilize the tube media
- autoclave the agar medium for plate production and then pour into sterile petri dishes
STERILIZATION AND THE AUTOCLAVE
When microbiological media has been made, it still has to be sterilized because of microbial contamination from air, glassware, hands, etc. Within a few hours there will be thousands of bacteria reproducing in the media so it has to be sterilized quickly before the microbes start using the nutrients up. The sterilization process is a 100% kill, and guarantees that the medium will stay sterile UNLESS exposed to contaminants by less than adequate aseptic technique to exposure to air.
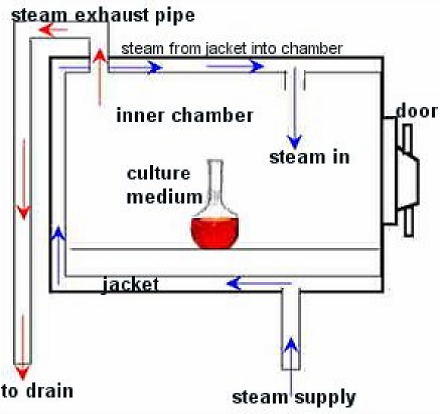

Media sterilization is carried out with the autoclave, basically a huge steam cooker. Steam enters into a jacket surrounding the chamber. When the pressure from the steam is at a certain point in the jacket, a valve allows the steam to enter the chamber. The pressure will go up over 15 pounds per square inch (psi): at this point the timer begins to count down--- usually for 15 minutes, depending on the type of media. The high pressure in a closed container allows the temperature to go above the highest temperature one could get by just boiling, around 121⁰C. Therefore, the parameters for sterilization with an autoclave are 121⁰C at >15 psi for 15 minutes. Fifteen minutes is the thermal death time for most organisms (except some really hardy sporeformers).
The prepared media is distributed in different ways, depending on the form one is making. Broths and agar deeps are dispensed into tubes and then sterilized. Agar slant tubes are sterilized and then the rack is tilted to allow the agar to solidify in a slanted fashion. Agar medium to be be poured into plates is sterilized in a flask, and then poured afterward. Not all media or solutions can be sterilized via an autoclave. Certain high-protein solutions such as urea, vaccines, and serum will denature in the extreme heat, and so they may have to be filter-sterilized without heat. You will be making slant and broth media, but not plate media in this lab.
MATERIALS NEEDED (per table)
- 2 plastic weigh boats
- 1 test tube rack
- 1-1 liter Erlenmeyer flask
- 1 pipet pump
- 1 graduated cylinder several nonsterile glass10 ml pipets
- 1 spatula
- 28 medium, nonsterile test tubes
- 1 jar agar powder 15 green caps
- 1 jar nutrient trypticase soy broth powder
- 15 yellow caps
- 1 magnetic stir bar
- 1 pipet disposal jar
THE PROCEDURE
Refer to the diagram below for the entire production:

- Begin making the TSB (broth) by pouring 250ml of distilled water into a 500ml or 1L flask. Put in the stir bar and turn on the stir plate so that the surface is just disturbed. Add 3.25 grams of the TSB powder to this flask and allow it to dissolve (will happen quickly). No heat need be applied at this stage.
- Once the powder is dissolved, pipet out 5ml green cap.
Green caps are always used for TSB.
- With the remaining solution (about 100ml) still stirring, add 2 grams of agar powder.
- The next step will require you to apply heat to the mixture. Before you do this, however, you should be aware that agar has a strong tendency to boil over when it reaches 100⁰C. Someone in your group should be watching the flask at all times once you see steam coming off of it. At the first sign that the mix is near boiling, REMOVE it from the hot plate (paper towels around the flask neck). DO NOT simply turn off the heat, letting the flask sit there. The metal plate retains a significant amount of heat, and turning off the heat will not prevent the flask from boiling over. Folded paper towels allow you to grasp the flask neck tightly, yet not burn your hand.
- Have you read step 4? OK, then you can turn on the heat to setting 9 (not High). Make sure that the magnetic bar is stirring the solution.
- Upon boiling, the agar dissolves, it will turn clear, deeper tan. Remove it from the heat and pipet out 5ml aliquots into 15 tubes for slants (will not be BE slants until removed from autoclave and tilted to the side to solidify). Cover the slant tubes with yellow caps. THE REST OF THE AGAR MEDIUM IN THE FLASK WILL BE POURED INTO 1 LARGE FLASK FOR THE CLASS.
From this point on, yellow caps will be used for nutrient agar slants.
Note
If the agar solidifies in the tip of the pipet, dispose of the pipet in the pipet jar and get another one. To prevent this from happening, either pipet out all the tubes at the same time, or leave the pipet in the flask of melted agar.
- Place all of the tubes you have pipetted out in the plastic autoclave racks on the instructor's table as well as the remaining of your melted agar. All agar slants go in one rack, broths in another rack, etc.
- Dispose of your used pipets in the pipet holder. These glass pipets are reusable, so don't throw them in the trash.
Questions
- What is a complex medium?
- Why are pH buffers added to the growth media for microbes?
- How can the temperature in the autoclave go above boiling temperature of 212 F?
- Why do you have boil the agar solution BEFORE dispensing it into tubes?
- At what temperature does agar solidify?
Contributors and Attributions
Jackie Reynolds, Professor of Biology (Richland College)

