1.10: Gram Stain
- Page ID
- 90557
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Explain the importance of Gram stains in health care and microbiology.
- Define "differential stain" and contrast with "simple stain."
- Identify the Gram stain as a type of differential stain
- Tell what the Gram stain tells us about different species of bacteria.
- Examine Gram-stained cells and interpret whether the cells are Gram-positive or Gram-negative.
- Identify cell morphology of bacteria.
- Describe the structure of the cell walls of Gram-positive cells.
- Describe the structure of the cell walls of Gram-negative cells.
- Identify structures of Gram-positive cell walls and Gram-negative cell walls in diagrams models of cell walls.
- Successfully conduct a Gram stain and differentiate cells as Gram-positive and Gram-negative.
- Name each stain/reagent of the Gram stain and explain its function and how it will interacts with Gram-positive and Gram-negative cell walls during the Gram stain procedure.
- Troubleshoot unsuccessful Gram stains and explain how errors might be fixed.
Differential Stains
Microbiologists commonly perform differential stains, as this allows them to gather additional information about the bacteria they are working with. Differential stains use more than one stain, and cells of different bacterial species can have different appearances based on their chemical or structural properties. Some examples of differential stains are the Gram stain, acid-fast stain, and endospore stain.
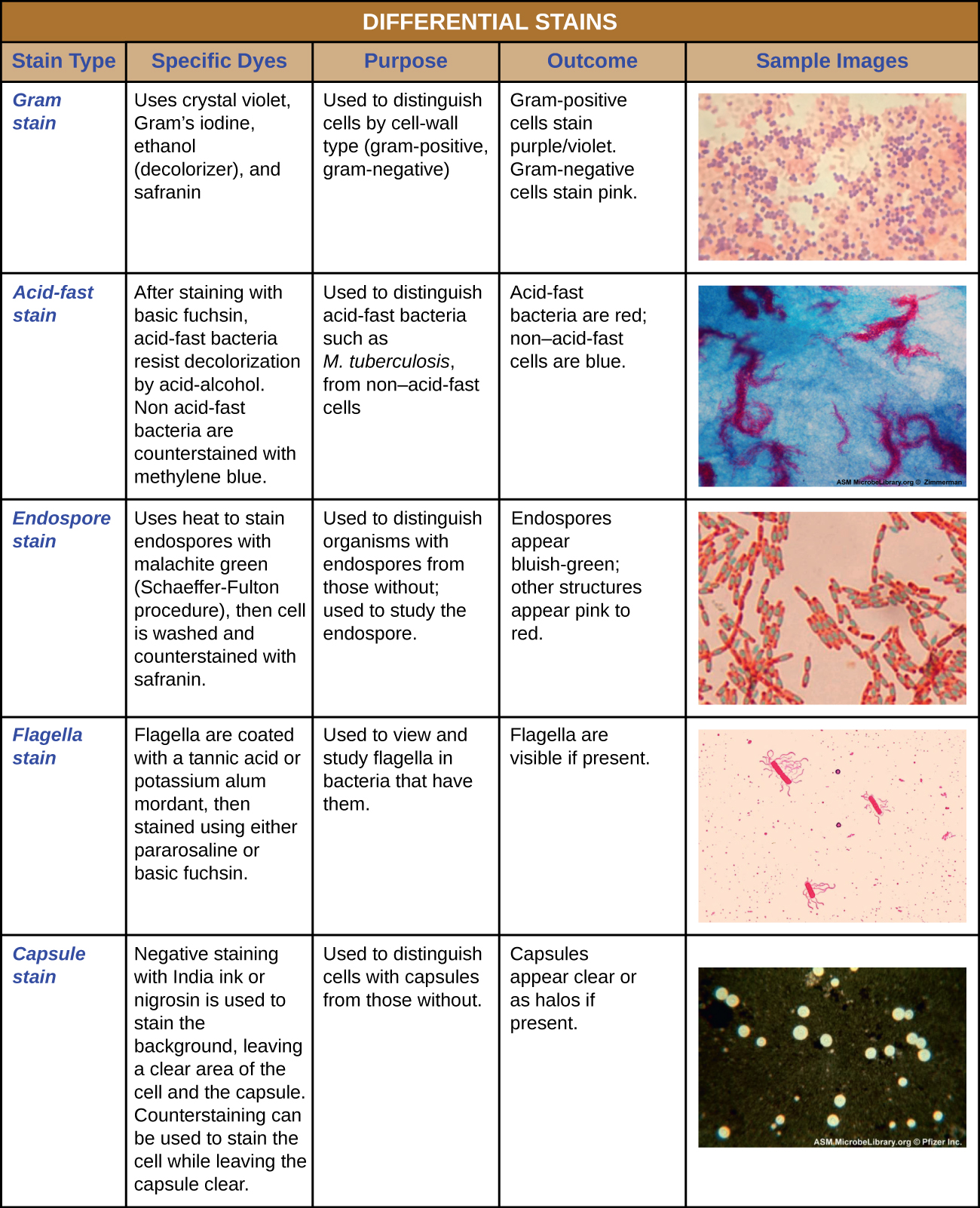
Table 1: Summary of some common differential stains used in microbiology. The Gram stain uses the following dyes/reagents: crystal violet, Gram's iodine, ethanol, and safranin. The Gram stain distinguishes cells by cell wall type (Gram-positive or Gram negative). Gram-positive cells stain purple/violet. Gram-negative cells stain pink. The acid fast stain uses the following dyes: basic fuchsin and methylene blue. The acid fast stain is used to distinguish acid fast bacteria (reveals the cell wall structure of certain types of bacteria beyond Gram-positive and Gram-negative). Acid fast bacteria appear read and non-acid fast bacteria appear blue. The endospore stain uses the following: heat, malachite green, and safranin. The endospore stain is used to distinguish between bacterial species that do and do not form endospores. Endospores (if present) appear bluish-green and all other cells/cell structures appear pink. The flagella stain uses the following dyes/reagents: tannic acid or potassium alum mordant, pararosaline or basic fuchsin. The flagella stain is used to visualize flagella (if present). The capsule stain uses the following dyes: India ink or nigrosin (stains the background, not cells) and a counterstain. The result shows the background as dark, the cells the color of the counterstain, and the capsule (if present) appearing clear. The capsule stain determines if a bacterial species has capsules or not. Capsules appear as transparent halos around dyed cells.
The Gram Stain Shows Differences in Cell Wall Structures
The Gram stain, developed by Christian Gram in 1884, is the most widely used differential stain in bacteriology. Most bacteria are divided into two major groups- Gram-positive bacteria and Gram-negative bacteria based on the cell wall composition. Knowing the Gram reaction of a clinical isolate (isolated bacterial species from a patient) can help the health care professional make a diagnosis and choose the appropriate antibiotic for treatment.
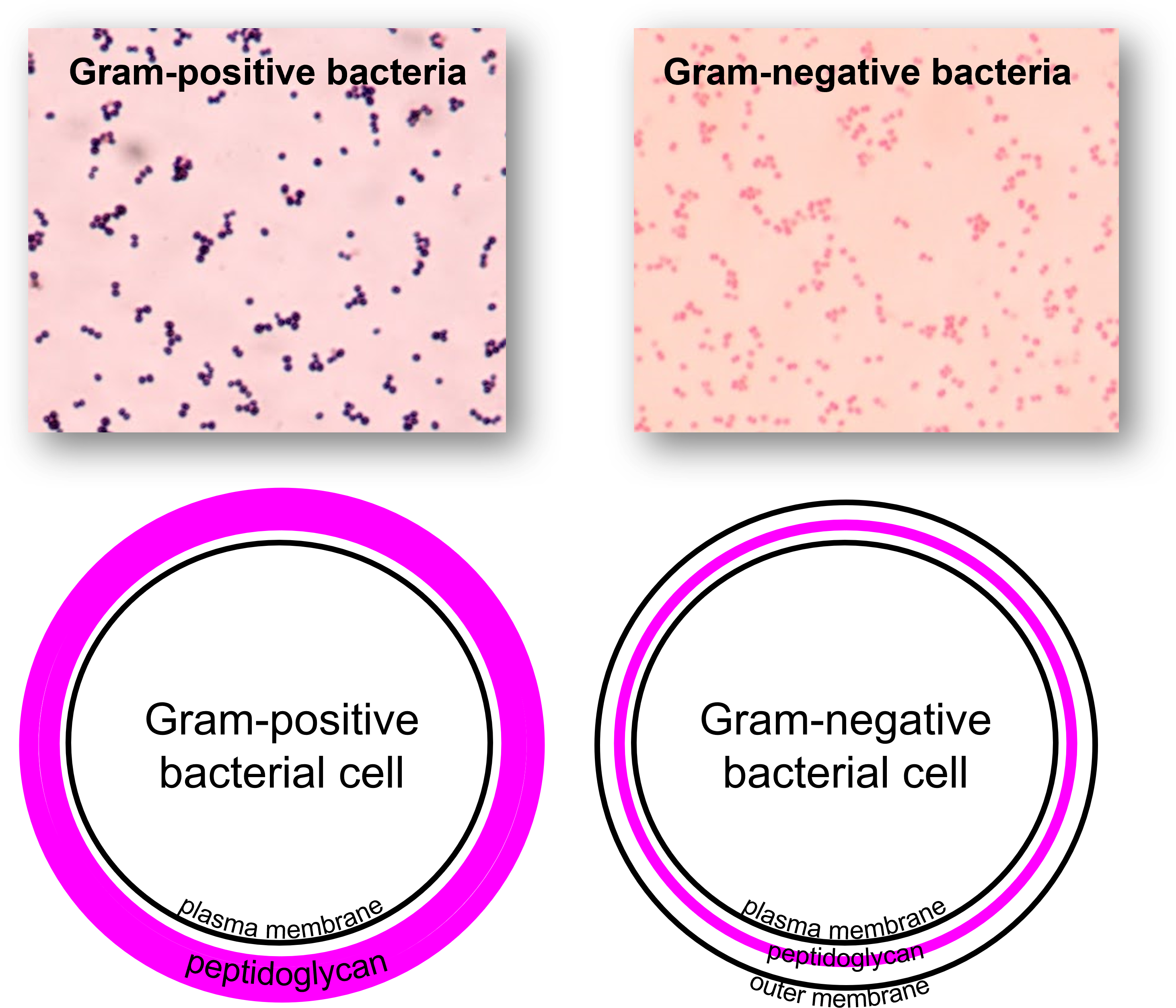
Figure 1: (Top) Microscopic images of bacteria stained with the Gram stain. (Top right) Gram-positive bacterial species stain purple with the Gram stain. (Top left) Gram-negative bacterial species stain pink with the Gram stain. (Bottom) Diagrams illustrating major structural components of Gram-positive bacterial cell walls and Gram-negative bacterial cell walls. (Bottom right) Gram-positive bacterial species have a thick layer of peptidoglycan outside of the plasma membrane. (Bottom left) Gram-negative bacterial species have a thin layer of peptidoglycan outside of the plasma membrane with an outer membrane outside of the peptidoglycan layer. Embedded in the outer membrane of Gram-negative cell walls are lipopolysaccharides (LPS) that are not shown in this diagram.
The results of the Gram stain reflect differences in cell wall composition. Gram-positive cells have thick layers of peptidoglycan (a substance composed of carbohydrates and protein subunits) in their cell walls. Gram-negative bacteria have very little peptidoglycan. Gram-positive bacteria also have teichoic acids, whereas Gram-negative bacteria do not. Gram-negative cells have an outer membrane that resembles the phospholipid bilayer of the plasma membrane. The outer membrane contains lipopolysaccharides (LPS), which are released as endotoxins when Gram-negative cells die. This can be of concern to a person with an infection caused by a Gram-negative organism.

Figure 2: Detailed view of the structure of bacterial cell walls for Gram-positive species and for Gram-negative species. (Left) Gram-positive cell walls consist of a thick layer of peptidoglycan (PG) outside of the cell's plasma membrane (PM) with teichoic acid (TA) and lipoteichoic acid embedded across the peptidoglycan layer. (Right) Gram-negative cell walls consist of a thin layer of peptidoglycan (PG) outside of the cell's plasma membrane (PM). Gram-negative cell walls have an outer membrane (OM) beyond the peptidoglycan layer containing lipopolysaccharide (LPS) as well as porin channels that enable some materials to pass across the outer membrane. In Gram-positive and Gram-negative cell wall types lipoprotein (LP) is also found.
Gram stains are best performed on fresh culture. Older cells may have damaged cell walls and not yield the correct Gram reaction. Also, some species are known as Gram-variable, and so both Gram-positive and Gram-negative reactions may be visible on your slide.
Although the vast majority of bacteria are either Gram-positive or Gram-negative, it is important to remember that not all bacteria can be stained with this procedure (for example, Mycoplasma sp., which have no cell wall, stain poorly with the Gram stain).
How the Gram Stain Works
The Gram stain uses four stains/reagents: crystal violet, Gram's iodine, ethanol, and safranin. Crystal violet (the primary stain), enters the peptidoglycan of all bacteria giving them a purple color. The next stain is Gram’s iodine, the mordant, which combines with the crystal violet to make a bigger complex in the peptidoglycan wall. The next step is the most critical. Ethanol is used as a decolorizer will remove the crystal violet/iodine complexes from the thin peptidoglycan layers of Gram-negative cell walls, but not the thick peptidoglycan layers of Gram-positive cell walls. The length of time given for the decolorization step with ethanol will be important in obtaining the best results from the Gram stain. After the decolorization step, Gram-positive cells will still hold onto the purple crystal violet color, but Gram-negative cells will lose the purple color and be transparent. We still want to see the Gram-negative cells and to be able to distinguish them from the Gram-positive cells. Safranin is called a counterstain since it is used to stain the now colorless Gram-negative cells a pink color.
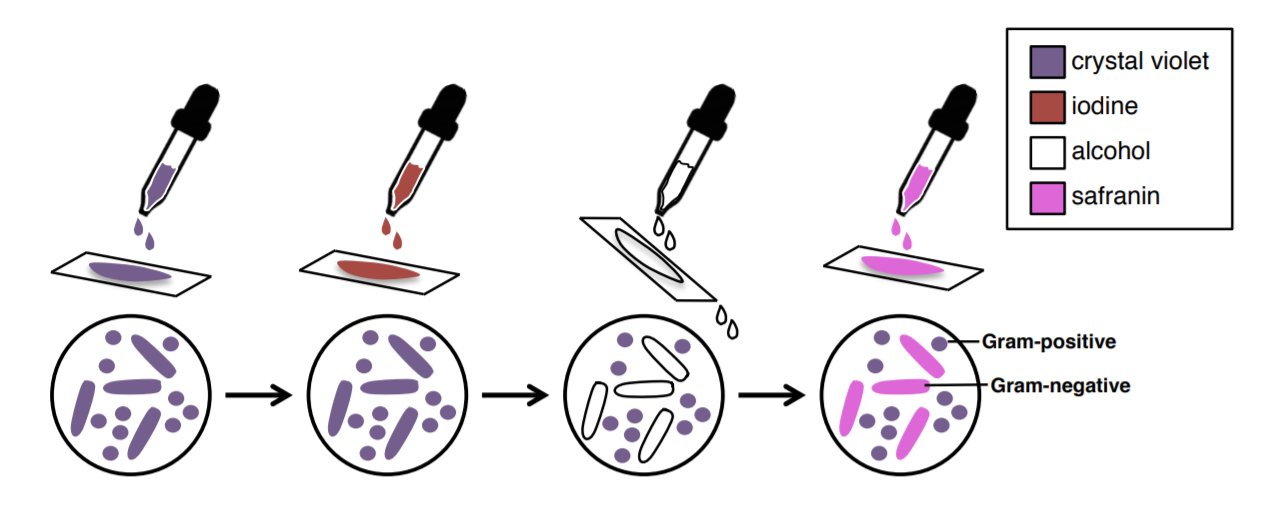
Figure 3: Overview of the Gram stain procedure and how each step changes cell color. (1) Applying crystal violet turns all cells purple. (2) Applying Gram's iodine does not change cell colors (all cells stay purple), but it will cause the crystal violet to firmly adhere to Gram-positive cell walls, but not Gram-negative cell walls. (3) The alcohol (ethanol) step is a decolorization step. Ethanol will remove crystal violet from Gram-negative cells, but not Gram-positive cells. After this step, Gram-positive cells will remain purple and Gram-negative cells will be transparent. (4) Safranin is a reddish-pink stain that will stain transparent cells (Gram-negative cells) pink. After this step, Gram-positive cells will still be purple and Gram-negative cells will be pink.
You conduct a Gram stain and when you observe the bacterial cells with the microscope, you observe pink colored cells. What does this tell you?
You conduct a Gram stain and when you observe the bacterial cells with the microscope, you observe purple colored cells. What does this tell you?
You conduct a Gram stain and when you observe the bacterial cells with the microscope, you observe purple colored cells and pink colored cells mixed together. What does this tell you?
The Gram stain has proven to be very useful in the identification of bacteria and in predicting which antibiotics are most likely to be effective to kill bacteria. There are some problems with the technique, however. If the procedure is not performed properly, the results may be erroneous. Thus, you will need to practice the technique until your results are satisfactory. There are some bacterial species, such as Mycobacterium tuberculosis or Legionella pneumophila that usually do not stain with the Gram stain at all. Nevertheless, this technique has become one that microbiologists rely heavily upon.
Table 2: Stains and reagents used during the Gram stain.
| Stain/Reagent use in the Gram stain | Color of the Stain/Reagent | Role of the Stain/Reagent | What the Stain/Reagent Does |
|---|---|---|---|
| crystal violet | purple | primary stain | stains all cells purple |
| Gram's iodine | amber | mordant | attaches to the crystal violet to form larger complexes that prevent crystal violet from leaving thick peptidoglycan layers in Gram-positive cell walls |
| ethanol | clear | decolorizer | removes crystal violet from thin peptidoglycan layers in Gram-negative cell walls leaving Gram-negative cells appearing transparent |
| safranin | red/pink | counterstain | colors transparent Gram-negative cells pink |
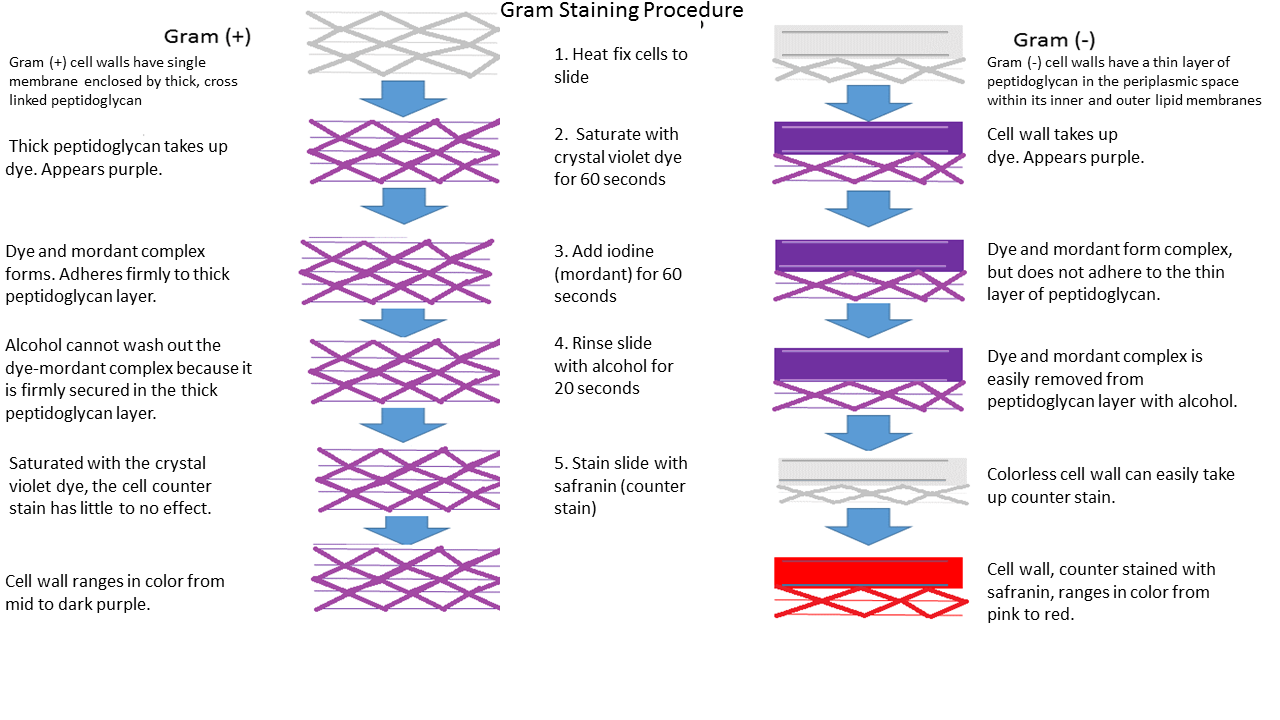
Figure 4: Gram-positive cell walls have a single membrane (the plasma membrane) enclosed by a thick, cross-linked peptidoglycan layer. Gram-negative cell walls have a thin layer of peptidoglycan between the plasma membrane and an outer membrane. When the Gram-positive cells and Gram-negative cells are saturated with crystal violet (first step of the Gram stain), they become purple. When Gram's iodine is applied to the cells, it causes the crystal violet to adhere firmly in the thick peptidoglycan layer of Gram-positive cell walls. In Gram-negative cell walls, the mordant does not firmly adhere the crystal violet within the thin peptidoglycan layer (even though iodine and crystal violet still react and form large complexes). In the third step of the Gram stain, the decolorizer, ethanol, is added to the cells. The ethanol removes the crystal violet from Gram-negative cells since crystal violet did not adhere strongly to the thin peptidoglycan layer and the cells become transparent. The crystal violet is not removed from the Gram-positive cells since the iodine-crystal violet complexes adhere to the thick peptidoglycan layer. Gram-positive cells remain purple. In the last step of the Gram stain, safranin is used as a counterstain to stain transparent cells (Gram-negative cells) pink. The end result is Gram-positive cells that are purple and Gram-negative cells that are pink.
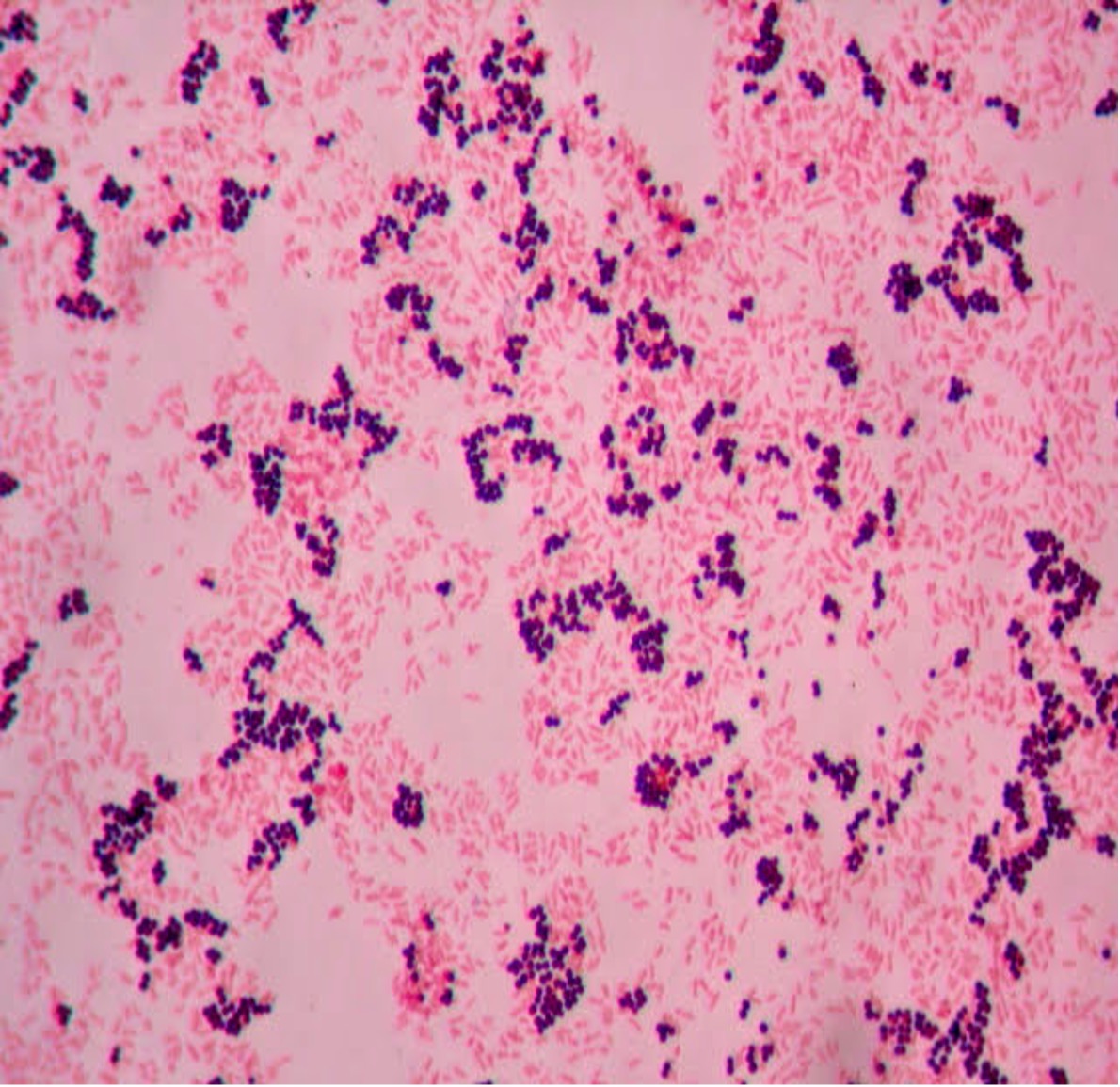
- How many species can you see in this Gram stain?
- What is the Gram (positive or negative) of the cells?
- What is the cell shape of the cells (see Bacterial Cell Morphology & Arrangement for assistance)?
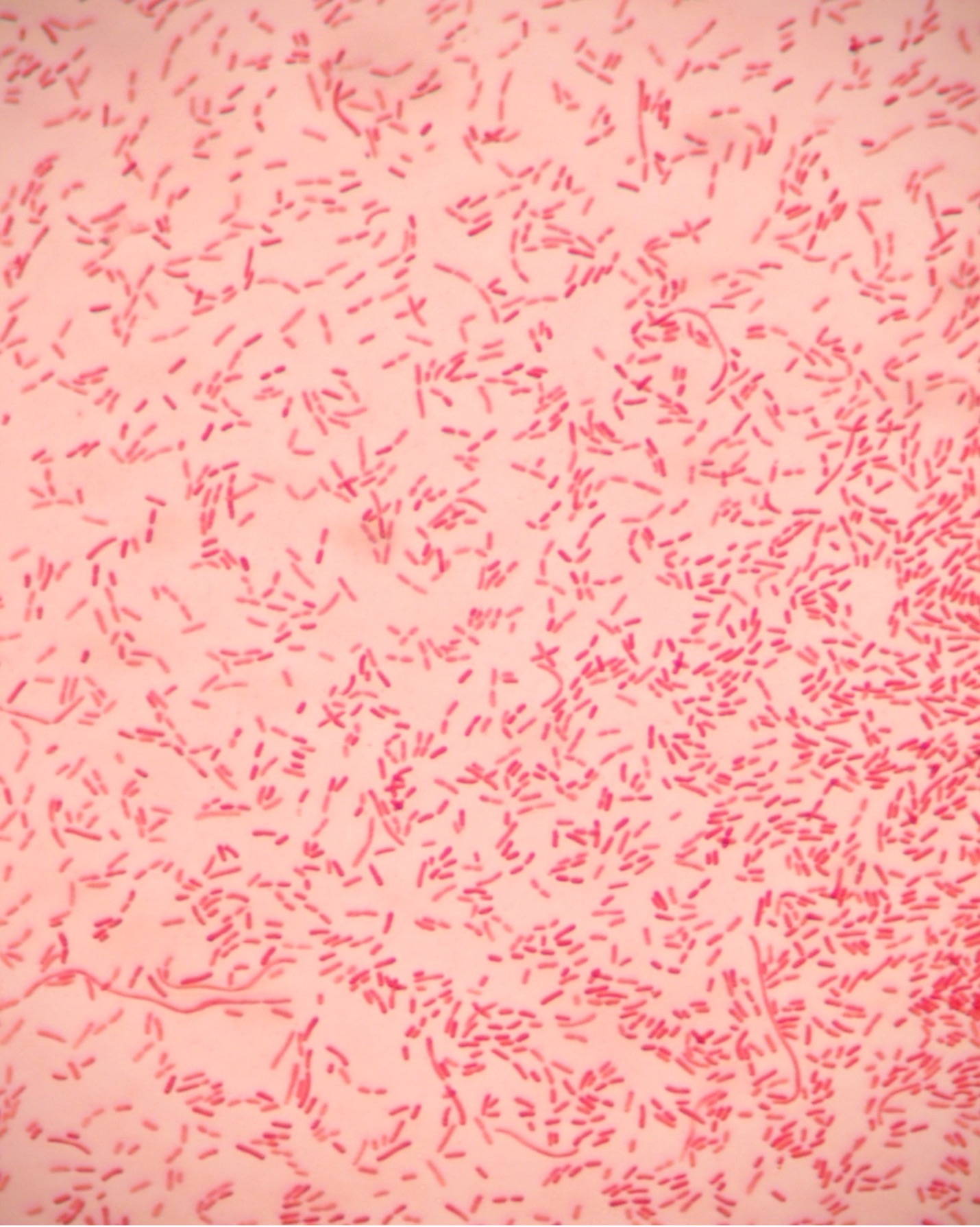
- How many species can you see in this Gram stain?
- What is the Gram (positive or negative) of the cells?
- What is the cell shape of the cells (see Bacterial Cell Morphology & Arrangement for assistance)?
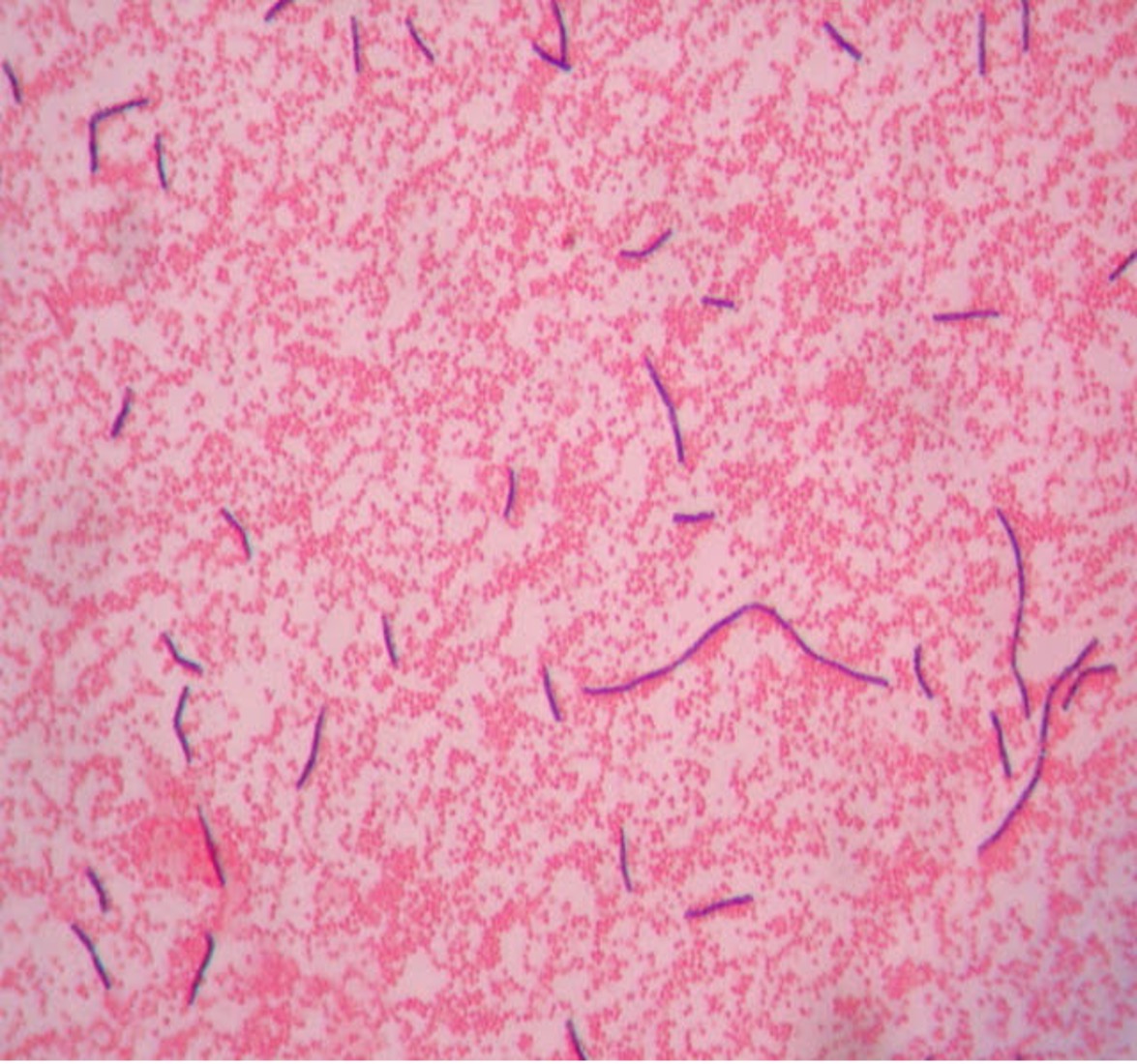
- How many species can you see in this Gram stain?
- What is the Gram (positive or negative) of the cells?
- What is the cell shape of the cells (see Bacterial Cell Morphology & Arrangement for assistance)?
Improving and Troubleshooting your Gram Stain Approach
Gram staining requires practice. It is an art as much as a science. If your results do not come out as they should, adjust your procedure to correct the problem for future stains.
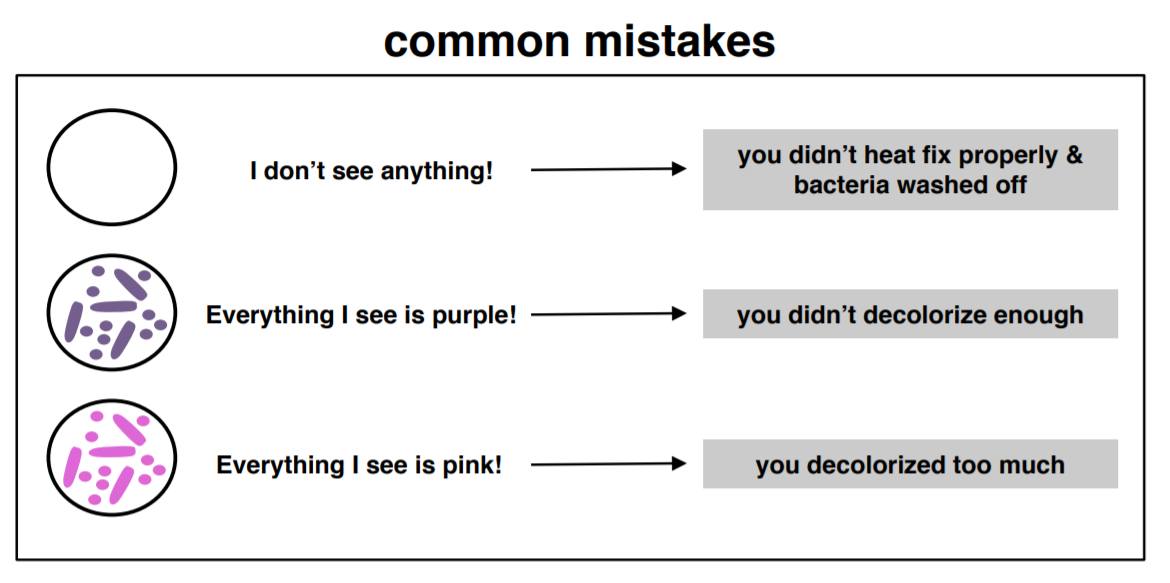
Figure 5: If you are staining a mixture of Gram-negative and Gram-positive bacteria and you do not see cells, see all cells purple, or see all cells pink, it is likely due to one of these common errors. (1) If you do not see anything (no cells are visible), it is likely because the smear was not properly heat-fixed and the bacteria washed off the slide during staining. (2) If you see all cells appear purple, it indicates that the decolorization step was too short. The next time you do a Gram stain, increase the time for decolorization. (3) If you see all cells appear pink, it indicates that the decolorization step was too long. The next time you do a Gram stain, decrease the time for decolorization.
Factors to control to obtain a proper Gram stain result:
- Smear preparation. Beginning students tend to make the smears too thick by putting too much inoculum on the slide. This will make it harder to properly decolorize the bacteria. Always put saline or water on the slide before or after adding bacteria when using a culture from solid medium (slant or plate). The amount of inoculum from a solid media should be a “pinpoint” amount. You should just barely touch the organisms you want to stain with your loop. NOTE: You do not need to put saline or water on the slide when making smears from a liquid media.
- Allow the smear to air dry. This means the slide must sit until it has no longer appears moist. Alternatively, you may use a slide warmer to dry your smear. The slide warmer will dry the slide faster. When using the slide warmer, do not take your eyes off the smear! As soon as the slide is dry, remove it from the slide warmer. If too much heat is used, the cell wall of the organisms may be distorted or rupture and release the crystal violet dye when it should retain it. Extreme overheating may cause the entire cell to lyse, and you will see nothing under the microscope.
- Heat fixing will adhere the bacteria to the slide and kill the bacteria. If you do not have any cells on the slide, likely the heat fixing step was skipped or not enough to fix the cells to the slide.
- Freshness and concentration of reagents. Reagents must be fresh, and concentrations must be accurate.
- Failure to drain slides before adding next reagent. After you wash a slide with water and before you add the next reagent, you must be sure to tilt the slide to allow excess water to drain off it. If you leave a bubble of water over the bacteria, the reagent you add may never reach the bacteria or it will be greatly diluted by the water present on the slide.
- Age of the bacterial culture. Older Gram-positive organisms lose their ability to retain the violet dye as they move out of the exponential growth phase.
- Timing of the decolorizing step. This is the most critical stage of the procedure. If the decolorizer is left on too long, Gram-positive organisms will appear Gram-negative. If the decolorizer is not left on long enough, Gram-negative organisms will appear Gram-positive. The thickness of the smear will dictate how long you will need to decolorize. It is impossible to give an exact time. You must decolorize one smear at a time and watch it closely. When the color begins to lift off the smear, you should wash off the decolorizer.
If you mix too aggressively, you will lose the bacterial morphology.
If you heat fix too little, the bacteria will wash off the slide. If you heat fix too much, you will cook the bacteria and denature them
Laboratory Instructions
Prepare a Bacterial Smear
You may find it helpful to draw a circle (wax pencil is best) on the opposite side of the slide where you will spread your smear. This will help you later in locating the smear. The wax pencil is better than a marker because it will not wash off easily from the glass.
If you are using a liquid culture, gently mix the culture until you get an even, cloudy mixture (it should look somewhat like skim milk). If you mix too aggressively, you will lose the bacterial morphology.
- Prepare a bacterial smear on the slide:
- If you are taking bacteria from a solid culture (slant or petri plate), place a small drop (only 1 drop) of saline, deionized (DI) water, or distilled water onto a microscope slide and use a loop to aseptically add bacteria to the water (avoid taking a large chunk of bacteria since the cells will be too dense to view individual cells). Use the loop to spread the bacteria in the water drop and to spread the water drop out to make it thinner (it will dry faster).
- If you are taking a bacteria from a a liquid culture (broth), place 1 or 2 loopfuls of bacteria directly onto a microscope slide (no saline or water added).
- Allow the slide(s) to air dry on the slide warmer (or air dry if a slide warmer is not available).
Heat Fix the Bacterial Smear
Once the liquid has completely evaporated on the surface of the slide, heat fix by passing the slide:
- Attach a wooden clip to the microscope slide to hold it.
- Pass the underside of the microscope slide through a flame three times.
- Allow the slide to cool and then continue with your staining protocol.
If you heat fix too little, the bacteria will wash off the slide. If you heat fix too much, you will cook the bacteria and denature them.
Gram Stain
- Use the slide(s) that you already prepared when creating bacterial smears and heat fixing (see above).
- Grip the microscope slide in wood clip over a waste container bucket.
- Primary stain: Add crystal violet (primary stain) onto the smear (there is no reason to cover the entire slide with stain). Allow stain to remain on the smear for 1 minute.
- Rinse the smear with deionized (DI) water or distilled water.
- Shake excess water off the smear.
- Mordant: Add Gram’s iodine onto the smear (there is no reason to cover the entire slide with iodine). Leave for 1 minute.
- Rinse with deionized (DI) water or distilled water.
- Shake excess water off the smear.
- Decolorizer: Tilt the slide at a 45° angle and let the ethanol run over surface of slide until no more crystal violet color comes out of the smear (time varies — no more than 5-15 seconds).
- Tilt the slide at a 45° angle and rinse the smear with deionized (DI) water or distilled water.
- Shake excess water off the smear.
- Counterstain: Add safranin onto the smear (there is no reason to cover the entire slide with stain) for 1 minute.
- Rinse with deionized water.
- Shake excess water off the smear.
- Gently blot (do not wipe) the excess water from the slide using bibulous paper.
- Place the stained slide on a microscope and examine. Be sure to begin at the lowest power (look for the purple and pink colors of the stains). Focus at lowest power and then increase one objective magnification at a time.
Results & Questions
- Draw the cells you observe after the Gram stain in the space above.
- Critique your cell drawings:
- Does the drawing accurately show how the cells appear when you look into the microscope (including cell shape and arrangement)?
- Does the drawing accurately show the cell size you see in the microscope?
- Does the drawing accurately show the color(s) you see in the microscope?
- After you examined your slide, did it show the Gram stain was successful? If you saw both Gram-positive and Gram-negative cells in the mixture of bacteria then your Gram stain was successful. If not, what did you see (or not see)?
- If your answer to question 3. indicated that your Gram stain was not successful, what might have occurred? See the section Improving and Troubleshooting your Gram Stain Approach for assistance.
- What was the cell shape of the Gram-positive cells you observed (see Bacterial Cell Morphology & Arrangement for assistance)?
- What was the cell shape of the Gram-negative cells you observed (see Bacterial Cell Morphology & Arrangement for assistance)?
- Staphylococcus aureus is the Gram-positive species you observed today. What does being "Gram-positive" mean about the cell wall structure of S. aureus?
- Escherichia coli is the Gram-negative species you observed today. What does being "Gram-negative" mean about the cell wall structure of E. coli?
- Some species of bacteria are Gram-variable. What does this mean?
- What might occur if the heat fixation step is skipped?
- What might occur if decolorization is too long?
- What might occur if decolorization is too short?
- What is the difference between a simple stain and a differential stain?
- Is the Gram stain a simple stain or a differential stain?
Attributions
- Cell wall peptidoglycan in Mycobacterium tuberculosis: An Achilles’ heel for the TB-causing pathogen by Arundhati Maitra et al. is licensed under CC BY 4.0
- Chapter Image: Gram stain 01.jpg by Y tambe is licensed under CC BY-SA 3.0
- General Microbiology Lab Manual (Pakpour & Horgan) by Nazzy Pakpour & Sharon Horgan is licensed under CC BY-SA 4.0
- Gram Stain.png by Paityn Donaldson is licensed under CC BY-SA 4.0
- Laboratory Exercises in Microbiology: Discovering the Unseen World Through Hands-On Investigation by Joan Petersen and Susan McLaughlin is licensed under CC BY-NC-SA 4.0
- Microbiology by OpenStax is licensed under CC BY 4.0
- Red Mountain Microbiology by Jill Raymond Ph.D.; Graham Boorse, Ph.D.; Anne Mason M.S. is licensed under CC BY-NC 4.0


