17.4B: Insecticides
- Page ID
- 5833
Inorganics
Inorganic compounds of arsenic, such as lead arsenate, have long been used against insect pests. However, these materials are highly toxic to nontarget organisms and persist in the environment. (Years after apple growers stopped using lead arsenate, high concentrations of lead can still be found in orchard soils.)
Botanicals
These are organic molecules (or mixtures) extracted from plants. Popular examples:
- pyrethrins
- rotenone
- nicotine
- azadirachtin. This extract from the neem tree affects insect growth and is discussed further under growth regulators.
Features:
- very toxic to insects
- relatively harmless to other organisms (except fish!)
- decompose readily so residues do not accumulate on crops or in the soil
- expensive, but you can get
- more bang for the buck by adding a synergist like piperonyl butoxide. Synergists have little toxicity themselves but enhance the effectiveness of the insecticide with which they are mixed.
Pyrethroids
Pyrethrins break down so rapidly in sunlight that they are of little use outdoors on crops. However, a number of synthetic pyrethrin-like substances — called pyrethroids — do not have this defect and are effective.
Example:
- permethrin (Ambush®, Pounce®)
Bacillus thuringiensis (B.t.)
This bacterium parasitizes many caterpillars. Its spores and/or mixtures of its protein toxins are now being used against a variety of insect pests. Examples:
- Dipel®
- Javelin®
- Agree®
These are all stomach poisons; that is, they must be ingested to work.

Chlorinated Hydrocarbons
DDT was the first of a long line of insecticides based on hydrocarbons with chlorine atoms replacing some of the hydrogen atoms. Its chemical name is dichloro, diphenyl, trichloroethane (see figure).
Some others:
- methoxychlor
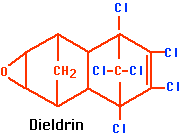
- dieldrin (see figure)
- dicofol (Kelthane®)
- endosulfan (Thiodan®, Phaser®)
DDT was introduced during World War II and, along with penicillin and the sulfa drugs, was responsible for the fact that this was the first war in history where trauma killed more people - combatants and noncombatants alike - than infectious disease.
DDT is effective against
- vectors of human diseases such as
- malaria and yellow fever (both transmitted by mosquitoes)
- plague (transmitted by fleas)
- many crop pests
Prior to the introduction of DDT, the number of cases of malaria in Ceylon (now Sri Lanka) was more than a million a year. By 1963 the disease had been practically eliminated from the island. However, growing concern about the hazards of DDT led to its abandonment there in the mid-1960s, and soon thereafter malaria became common once again.
DDT was especially effective against malarial mosquitoes because of its persistence and resistance to breakdown in the environment. One or two sprays a year on the walls of homes kept them free of mosquitoes. But DDT has several serious drawbacks.
Insecticide resistance
As early as 1946, Swedish workers discovered populations of houseflies resistant to DDT. This was quickly followed by many other reports of developing resistance. Other chlorinated hydrocarbons (like dieldrin and methoxychlor) were developed as substitutes, but in time insects developed resistance to these as well.
Persistence
DDT is stable and fat soluble. These properties cause it to accumulate in fat tissue. People who were heavily exposed to DDT (during its manufacture or application) often showed concentrations of DDT in their fat 1000 times higher than that in their blood. Even these high levels were probably of little harm to the workers. In the early stages of exposure, the blood levels of DDT (and its metabolite DDE) rise rapidly at first and then reach a steady level. From that point on, the body excretes it as fast as it acquires it.
Biomagnification
Although no harmful effects from average exposures to DDT have been seen in humans, DDT and other chlorinated hydrocarbons have been shown to harm other species, such as fishes, earthworms, and robins. The hazard of DDT to nontarget animals is particularly acute for those species living at the top of food chains. Carnivores at the ends of long food chains (e.g., ospreys, pelicans, falcons, and eagles) once suffered serious declines in fecundity and hence in population size because of this. High levels of chlorinated hydrocarbons interfere with forming eggshells of normal thickness.
| Species | Location | Average Concentration of DDE in Eggs (ppm) |
Reduction in Shell Thickness |
|---|---|---|---|
| Peregrine falcon | Alaskan tundra (north slope) | 889 | -21.7% |
| Peregrine falcon | Central Alaska | 673 | -16.8% |
| Peregrine falcon | Aleutian Islands | 167 | -7.5% |
| Rough-legged hawk | Alaskan tundra (north slope) | 22.5 | -3.3% |
| Gyrfalcon | Seward Peninsular, Alaska | 3.88 | 0 |
Another group of nontarget victims of DDT (and other pesticides) are insects that prey upon insect pests; that is, the natural enemies of the pests. Killing these has serious ecological and economic effects. For example, once apple growers began controlling pests with DDT, they quickly found their orchards being attacked by scale insects and mites. The reason: DDT had killed off their natural enemies.
 Organophosphates
Organophosphates
The organophosphates, e.g., parathion (right), are related to the nerve gases developed during World War II. They react irreversibly with the enzyme acetylcholinesterase, which is responsible for inactivating acetylcholine (ACh) at neuromuscular junctions and at certain synapses in the central and peripheral nervous systems.
Some other examples:
- malathion
- diazinon
- phosmet (Imidan®)
- chlorpyrifos (Lorsban®)
Some of the organophosphates are very toxic. Parathion, for example, is 30 times more toxic than DDT. Each year organophosphates poison thousands of humans throughout the world, causing hundreds of deaths. Medical personnel caring for poisoning victims are also at risk. They may be seriously poisoned by the excretions of, and even the vapors emanating from, their patients.
This table gives the LD50 values for some insecticides. In each case, the chemical was fed to laboratory rats. Note that the lower the LD50, the more toxic the chemical.
| Chemical | Category | Oral LD50 in Rats (mg/kg) |
|---|---|---|
| Aldicarb ("Temik") | Carbamate | 1 |
| Carbaryl ("Sevin") | Carbamate | 307 |
| DDT | Chlorinated hydrocarbon | 87 |
| Dieldrin | Chlorinated hydrocarbon | 40 |
| Diflubenzuron ("Dimilin") | Chitin inhibitor | 10,000 |
| Malathion | Organophosphate | 885 |
| Methoprene | JH mimic | 34,600 |
| Methoxychlor | Chlorinated hydrocarbon | 5,000 |
| Parathion | Organophosphate | 3 |
| Piperonyl butoxide | Synergist | 7,500 |
| Pyrethrins | Plant extract | 200 |
| Rotenone | Plant extract | 60 |
Unlike chlorinated hydrocarbons,
- Organophosphates break down quickly in the environment, and thus residues on crops are less likely to be a problem.
- They are not stored in animal tissue, so biomagnification has not been a problem either.
For these reasons, their use has greatly reduced the hazard to nontarget species like ospreys and eagles (at the price of a much greater hazard to humans). Development of resistance is just as much a problem as it is with the chlorinated hydrocarbons. The carbamates were introduced in an attempt to keep ahead.
 Carbamates
Carbamates
Carbamate insecticides are also inhibitors of acetylcholinesterase, but their action is reversible.
Some examples:
- carbaryl (Sevin®)
- aldicarb (Temik®)
- methomyl (Lannate®)
Features:
- These compounds are rapidly detoxified and excreted so their risk to warm-blooded animals is less than the other agents we have looked at.
- They are degraded rapidly in the environment so persistence is not a problem.
- They are, however, a danger to many useful insects, especially honeybees.
Growth Regulators
The members of this diverse group interfere in one way or another with insect development. Although most insect growth regulators do not affect adults, for many pests, it is the larval stages that are the most destructive.
Chitin Inhibitors
These substances, diflubenzuron (Dimilin®) is an example, interfere with the synthesis of chitin, the material that makes up the insect exoskeleton. It seems to have very low toxicity for vertebrates, but is harmful to crustaceans as well as insects. Its effect on fungi, which also synthesize chitin, needs to be studied.
Molting Disruptors
Insects must periodically shed their exoskeleton — called molting — in order to grow. Each molt is triggered by a steroid hormone called ecdysone.
A few synthetic ecdysone
- agonists, e.g.,
- tebufenozide (Confirm®)
- methoxyfenozide (Intrepid®)
- inhibitors, e.g., azadirachtin (Neemix®)
are now used as insecticides.
Juvenile Hormone (JH)
In some insects, the final molt produces an adult that looks pretty much like the earlier larval stages. In others, like moths and butterflies, the final molt of the larva (caterpillar) produces a pupa. After a period of metamorphosis, the adult moth emerges.
Ecdysone triggers larva-to-larva molts as long as another hormone, called juvenile hormone (JH), is present. In its absence, ecdysone promotes the pupa-to-adult molt. Thus normal metamorphosis seems to occur when the output of JH diminishes spontaneously in the mature caterpillar.
When solutions of JH are sprayed on mature caterpillars, or on the foliage upon which they are feeding, their normal development is upset. This raises the possibility of using JH as an insecticide (one that might avoid the problem of developing resistance).
As it turns out, JH is too unstable to be practical, but some synthetic JH mimics, e.g.,
- methoprene (Altosid®)
- pyriproxyfen (Esteem®, Knack®, Distance®)
- diofenolan
are now being used.
Precocenes
These are substances, first discovered in plants, that damage the corpora allata thus preventing juvenile hormone (JH) from doing its normal job. Applied to early larval stages, precocenes induce premature or precocious metamorphosis (like that induced by the surgical removal of the corpora allata). Not only does precocious metamorphosis cut short the destructive larval phase of the insect, but the adults are abnormal (besides being small). The females, for example, are sterile (because JH is needed for development of the ovaries).
Summary
It is often said that the evolution of insecticide resistance requires the introduction of ever more powerful insecticides. But what is really needed is a new substance that attacks some other chink in the insect's armor. Whether gram for gram the new substance is more or less toxic to the insect (and whether its LD50 for mammals is lower or higher) is quite a separate issue.


