12.9: Immunotherapy of Cancer
- Page ID
- 5092
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Most cancer patients are treated with some combination of surgery, radiation, and chemotherapy. Radiation and chemotherapy have the disadvantage of destroying healthy as well as malignant cells and thus can cause severe side-effects. What is needed are more precisely-targeted therapies. One long-held dream is that the specificity of immune mechanisms could be harnessed against tumor cells. This might use the patient's own immune system or the transfer of antibodies or T cells from an outside source (i.e. passive immunization). Ideally, these agents would be targeted to molecules expressed on the cancer cells but not on healthy cells. However, such tumor-specific antigens have been hard to find, and so many of the immune agents now in use do target healthy cells as well.
Immunostimulants
There is considerable evidence that cancer patients have T cells that are capable of attacking their tumor cells. In fact, it may be that the appearance of cancer is a failure of immune surveillance: the ability of one's own immune system to destroy cancer cells as soon as they appear. But what to do if they fail? Immunostimulants are nonspecific agents that tune-up the body's immune defenses. There have been some successes with
- injecting adjuvant-like agents directly into the tumor. The only one that succeeds often enough to remain in use is the bacterial preparation BCG. Introduced into the bladder, it can help eradicate early-stage bladder tumors.
- Oral therapy with levamisole, a drug widely-used for deworming (people as well as animals), has been used to treat a variety of cancers but with inconsistent results.
- interleukin-2 (IL-2), a potent growth factor for T cells;
- alpha-interferon (IFN-α)
Cancer Therapy with Monoclonal Antibodies
A number of monoclonal antibodies show promise against cancer, especially cancers of white blood cells (leukemias, lymphomas, and multiple myeloma). Some examples:
- Rituximab (trade name = Rituxan®). Used to treat B-cell lymphomas. The CD20 molecule to which it binds is present on most B-cells, healthy as well as malignant, but over the months following treatment, new healthy B cells are formed from precursors that do not have CD20 and thus were not destroyed by the treatment.
- Trastuzumab (trade name = Herceptin®). Binds HER2, a growth factor receptor found on some tumor cells (some breast cancers, lymphomas). The only monoclonal so far that seems to be effective against solid tumors.
- Alemtuzumab (MabCampath®). Binds to CD52, a molecule found on white blood cells. Has produced remission of chronic lymphocytic leukemia.
- Lym-1 (Oncolym®). Binds to the HLA-DR-encoded histocompatibility antigen that can be expressed at high levels on lymphoma cells.
- Bevacizumab (Avastin®). Binds to vascular endothelial growth factor (VEGF) thus blocking its action and depriving the tumor of its blood supply.
- Cetuximab (Erbitux®). Used to treat colorectal cancers.
- A monoclonal antibody against CD47. CD47 is a cell-surface protein expressed at high levels in many different human cancers. CD47 blocks any effort that macrophages and dendritic cells might make to phagocytose the cancer cells; that is, CD47 is a "don't eat me" signal. A variety of human cancers transplanted into immunodeficient mice have their growth suppressed and metastases prevented when the mice are given a monoclonal antibody against CD47 thus unleashing the ability of phagocytes to destroy the cancer cells. The success in mice will soon lead to clinical trials in humans.
- Ipilimumab (Yervoy®). Unlike the other monoclonals listed here, ipilimumab acts as an immunostimulant. It does so by binding to the CTLA-4 molecules on the T cell so that they cannot bind to the B7 molecules on the antigen-presenting cell. This frees the T cell's CD28 molecules to bind B7 thus receiving the stimulatory "signal 2" from the antigen-presenting cell. Ipilimumab was approved by the U.S. Food and Drug Administration on 25 March 2011 for use against metastatic melanoma. Because its double-negative effect works to enhance the body's overall T-cell responses, it may well turn out to be useful against other cancers as well (and explains some of the autoimmune-like side effects it produces). It also provides perhaps the best evidence yet for the existence of immune surveillance; that is, the presence in the patient's body of an innate population of T cells specific for the tumor.
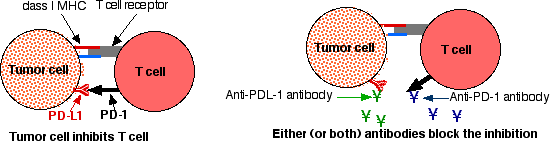
- Pembrolizumab and nivolumab
- Blinatumomab is a synthetic monoclonal antibody each arm of which carries a binding site with a different specificity:
- one arm binds to CD19, an antigen found on the surface of B cells and B-cell lymphomas
- the other arm binds to CD3, a cell-surface molecule on T cells, including cytotoxic T lymphocytes (CTLs)
By forming a bridge between CD3 and CD19, blinatumomab is able to attach T cells to B cells and activate the T cells to kill the B cells. Early clinical trials with blinatumomab on a small number of patients appear quite promising. Modest doses of the drug produced partial and, in a few cases, complete regression of their lymphoma.
Immunotoxins
A major problem with chemotherapy is the damage the drugs cause to all tissues where rapid cell division is going on. What is needed is a "magic bullet", a method of delivering a cytotoxic drug directly and specifically to tumor cells, sparing healthy cells. Such a magic bullet would have two parts - a monoclonal antibody specific for the cancer cell attached to a cytotoxic drug or toxin that kills the cell once it gets inside.
Some two dozen immunotoxins are in clinical trials. Two that have already received FDA approval:
- Adcetris®. The vedotin is attached to the monoclonal antibody by a bridge that is cleaved once the conjugate is safely inside the tumor cell releasing the toxin to do its work there. In one trial, 73% of the patients with Hodgkin's lymphoma went into remission.
- a monoclonal antibody that binds CD30, a cell-surface molecule expressed by the cells of some lymphomas but not found on the normal stem cells needed to repopulate the bone marrow.
- vedotin, a drug that blocks mitosis by preventing the polymerization of tubulin (needed to form the mitotic spindle).
- Kadcyla® The DM1 is attached to the monoclonal antibody by a bridge that is cleaved once the conjugate is safely inside the tumor cell releasing the toxin to do its work there. Kadcyla® prolongs survival in women whose breast cancer over-expresses HER2 (about 20% of breast cancer cases).
- Trastuzumab (Herceptin®), the monoclonal antibody against HER2 listed above;
- DM1, another drug that inhibits mitosis by preventing the polymerization of tubulin (needed to form the mitotic spindle).
Radioimmunotherapy
Monoclonal antibodies against tumor antigens can also be coupled to radioactive atoms. The goal with these agents is to limit the destructive power of radiation to those cells (cancerous) that have been "fingered" by the attached monoclonal antibody. Examples:
- Zevalin®. This is a monoclonal antibody against the CD20 molecule on B cells (and lymphomas) conjugated to either
- the radioactive isotope indium-111 (111In) or
- the radioactive isotope yttrium-90 (90Y)
- Bexxar® (tositumomab). This is a conjugate of a monoclonal antibody against CD20 and the radioactive isotope iodine-131 (131I). It, too, is designed as a treatment for lymphoma. Although both Bexxar® and Zevalin® kill normal B cells, they don't harm the B-cell precursors because these do not express CD20. So, in time, the precursors can repopulate the body with healthy B cells.
On 3 February 2005, the New England Journal of Medicine reported that 59% of patients with a B-cell lymphoma were disease-free 5 years after a single treatment with 131I-tositumomab (a treatment that was relatively free of the nasty side-effects, e.g., hair loss, of conventional chemotherapy).
Adoptive Cell Therapy (ACT)
Tumor destruction is done by cells. Antibodies may help, but only by identifying the cells to be destroyed, e.g., by macrophages. But T cells, e.g., cytotoxic T lymphocytes (CTL), are designed to destroy target cells. What about enlisting them in the fight?
Tumor-Infiltrating Lymphocytes (TIL)
Solid tumors contain lymphocytes that are specific for antigens expressed by the tumor. For many years, Steven A. Rosenberg and his associates at the U. S. National Cancer Institute have tried to enlist these cells in cancer therapy.
On September 19, 2002, he reported his most promising results at that time. The procedure:
- Isolate T cells — both CD4+ T-helper cells and CD8+ cytotoxic T lymphocytes (CTL) from samples of the tumor (melanoma)
- Test them in vitro to find the most efficient killers of the melanoma cells.
- Grow large numbers of them in culture (using the powerful T-cell growth factor IL-2).
- Treat the patient with modest doses of cytotoxic drugs to reduce — but not destroy — the bone marrow (called nonmyeloablative conditioning).
- Reintroduce the mix of Th cells (CD4+) and CTL (CD8+) into the patient (along with IL-2).
The results:
- The infused cells usually took up long-term residence.
- In 10 of 13 patients, their melanoma cells — including all metastases — regressed either partially or completely.
In a few cases, the TIL seemed to be reacting to tumor-specific antigens, but in most the target seems to have been antigens expressed by all melanin-containing cells. Evidence:
- Four patients lost normal melanocytes from their skin leaving white patches.
- One patient developed inflammation of the uvea, the coat of melanin-containing cells within the eye.
Adoptive transfer of a clone of the patient's own tumor-antigen-specific T cells
The 19 June 2008 issue of the New England Journal of Medicine (Naomi Hunder et al) carried a report describing the successful treatment of a man with metastasized melanoma using his own T cells. The procedure:
- His leukocytes were harvested and a mixed culture was prepared containing
- antigen-presenting dendritic cells.
- a peptide from the antigen NY-ESO-1. NY-ESO-1 is a protein that is produced by several types of tumors (e.g., melanoma, lung and breast cancers) but is not expressed by normal cells (except those in the testis).
- The patient's own T cells.
- After repeated stimulation with the antigen, responding cells were cloned by limiting dilution.
- One (of four) antigen-reactive cells was then expanded in culture until
- 5 billion (5 x 109) identical anti-NY-ESO-1 CD4+ T cells were available to infuse into the patient.
The result: complete regression of each metastatic clump of melanoma cells, and the patient has remained free of this lethal cancer for two years since this treatment.
Adoptive transfer of genetically-modified T cells
Genetically engineered with a T-cell Receptor
On April 20, 2006, the Rosenberg group reported some success with melanoma patients using a modification of the TIL procedure.
- The patient's T cells were removed and treated with a retroviral vector containing the αβ T-cell receptor (TCR) specific for a melanoma antigen.
- Large numbers of these were grown in culture.
- After nonmyeloablative conditioning to "make room" for them, the genetically-modified lymphocytes were infused into the patient.
- This application of gene therapy succeeded in eliminating the metastases and providing a disease-free period of two years in two patients.
Genetically engineered with a Chimeric Antigen Receptor (CAR)
The 10 August 2011 online version of the New England Journal of Medicine carried a report by Porter, D., et al. on their results with one (of three) patients treated for chronic lymphocytic leukemia (CLL) with an infusion of his own genetically-modified T cells.
The patient's malignant B cells expressed the surface antigen CD19 just as normal B cells do.
T cells were harvested from his blood and later treated with a vector encoding the antigen-binding site of an anti-CD19 antibody along with two other costimulatory molecules. The result: some 5% of these T cells expressed this synthetic antibody (called a chimeric antigen receptor or CAR) and were activated when they bound CD19 with it (rather than with their T cell receptor (TCR) which they would normally use).
Injected back into the patient, they proliferated by some 1000-fold and persisted for months. During this period, they eliminated all his malignant B cells (as well as his normal B cells). At the time of the report (10 months after treatment), he continued to be free of his cancer. Lacking normal B cells as well, he needed periodic infusions of immune globulin to keep infections at bay.
"One swallow does not make a summer", but these results give hope that in time immunotherapy will become an effective weapon against cancer.
Cancer Vaccines
Any response of the patient's own immune system – immune surveillance – has clearly failed in cancer patients. The purpose of cancer vaccines is to elicit a more powerful active immunity in the patient. Several approaches are being explored.
Patient-Specific Cancer Vaccines
Patient-Specific Dendritic-Cell Vaccines
Dendritic cells are the most potent antigen-presenting cells. They engulf antigen, process it into peptides, and "present" these to T cells.
To make a dendritic-cell vaccine,
- Harvest dendritic cells from the patient.
- Expose these in vitro to antigens associated with the type of tumor in the patient.
- The antigens are found in normal – as well as cancerous – cells of that tissue (e.g., tyrosinase in melanocytes, prostatic acid phosphatase [PAP] in prostate cells).
- They may be fused with a stimulatory molecule such as granulocyte-macrophage colony-stimulating factor (GM-CSF)
- Inject these "pulsed" dendritic cells back into the patient.
- Hope that they elicit an strong cell-mediated immune response, e.g. by cytotoxic T lymphocytes (CTL).
On 29 April 2010 the U.S. Food and Drug Administration approved the first anti-cancer vaccine: a patient-specific dendritic-cell vaccine for use against advanced prostate cancer. The vaccine, called sipuleucel-T (Provenge®), is produced by pulsing the patient's dendritic cells with a fusion protein coupling prostatic acid phosphatase [PAP] with GM-CSF.
Patient-Specific Tumor-Antigen Vaccines
The antigens in these vaccines are taken from the patient's own tumor cells.
- Harvest some tumor cells from the patient.
- Ship them to a company that will use them to make complexes with adjuvant materials.
- The complexes are returned to be injected into the patient.
Several of such vaccines are currently in clinical trials.
Tumor-Antigen-Specific Vaccines
These vaccines are used to immunize the patient with an antigen universally expressed by tumors of that type (but not by normal cells) mixed with some form of adjuvant that will enhance the response.
Examples:
- Many cancer patients mount an immune response — both antibody-mediated and cell-mediated — against the tumor (and testis) antigen NY-ESO-1. Deliberate immunization with this protein (plus an adjuvant) boosts this response and has shown some promise in early clinical trials. (Cells in the testis do not express HLA antigens, so are not at risk from attack by NY-ESO-1-specific cytotoxic T lymphocytes).
- MAGE-A3 is another protein common on cancer cells. A vaccine using MAGE-A3 — along with an adjuvant — is in Phase III clinical trials to assess its effectiveness against melanoma and lung cancer.
- HER2 is a protein over-expressed on 20–30% of breast cancers. NeuVax® is a vaccine that contains a peptide of HER2 along with recombinant GM-CSF as an adjuvant. It stimulates the formation of cytotoxic T lymphocytes (CTLs) that attack cells expressing HER2 and has shown promise in clinical trials.
Unlike patient-specific vaccines, these vaccines can be mass-produced for use in anyone with the appropriate tumor.
Combining Procedures #3 and #4
While tumors are immunogenic in the patient who carries them, they are only weakly so. In the hopes of improving cancer immunotherapy, clinical trials are now proceeding to test the efficacy of combining potent patient-specific cancer immunization with treating the patient with large numbers of cultured cancer-antigen-specific T cells that result.
The patient is repeatedly immunized with his or her own cancer cells along with a strong adjuvant (e.g., GM-CSF) followed by harvesting the patient's leukocytes and growing large numbers of them in the laboratory before infusing them into the patient along with interleukin-2. This combined approach — which generates large numbers of patient-cancer-specific killer T cells — has been tested against kidney and one type of brain cancer with promising results.
Blood Cancers
Cancers of blood cells, leukemias and lymphomas, arise in the bone marrow — the source of all blood cells.
One approach to curing leukemia is to treat the patient with such high doses of chemotherapy and radiation that not only are the leukemic cells killed, but the patient's bone marrow is destroyed. If the patient is to survive the treatment, called "myeloablative conditioning", he or she must be given a transplant of hematopoietic stem cells — the cells from which all blood cells are formed.
The stem cells can be
- an autograft; that is, from bone marrow harvested from the patient and stored before treatment begins. In this case, however, the marrow must also be treated to purge it of all cancer cells it may contain before it is returned to the patient. This sometimes fails.
- an allograft; that is, cells harvested from another person, usually a family member sharing the same major histocompatibility molecules.
Allografted hematopoietic stem cells also sometimes fail to cure, but in that case it is because not all of the patient's leukemic cells were destroyed. However, an infusion of T lymphocytes from the blood of the same donor that provided the cells can finish off the job.
This effect is called the graft-versus-leukemia effect.
However, most (if not all) of the donor T cells are probably attacking normal cell surface molecules, not tumor-specific ones. (Even if the donor and recipient are matched for the major histocompatibility molecules, there will be minor ones that elicit a rejection response.)
So the patient may also suffer life-threatening graft-versus-host disease (GVHD).
The graft-versus-leukemia effect lays the foundation for an approach that has shown considerable promise against various blood cancers and even some solid (e.g., kidney) tumors.
- The patient is treated to kill some — but not all — of the bone marrow cells (nonmyeloablative conditioning).
- Instead of using high doses of radiation to the entire body and chemotherapy, only the lymphoid organs (spleen, thymus, lymph nodes) are irradiated (called "total lymphoid irradiation").
- Antithymocyte globulin can also be given.
- Even though this leaves some cancer cells, it makes it possible for allogeneic bone marrow stem cells to take up long-term residence in the recipient (just as immunosuppression allows kidney transplants, etc. to avoid rejection by the recipient).
- This is followed by an infusion of T cells from the same donor. These can then go to work against the cancer cells without being threatened with rejection by the host.
- Once again, though, they will also attack normal cells of the recipient usually causing graft-versus-host disease (GVHD). However, this promises to be milder than that following myeloablative conditioning — perhaps because repeated small doses of radiation favors the survival of natural killer (NK) cells, and these appear to protect against GVHD.
In mice, the graft-versus-leukemia effect can be enjoyed without the downside of GVHD by including extra-large numbers of regulatory T cells (Treg cells) in the bone marrow infusion. Whether this approach could be helpful for humans remains to be seen.
Virotherapy
It has long been known that viral infections can occasionally (and unpredictably) cause tumors to regress. A number of viruses have been studied in the hope of developing a reliable therapy. On 27 October 2015, the U.S. FDA approved T-VEC (Imlygic®) for the treatment of melanoma. T-VEC is a mutated and engineered Herpes Simplex Virus (HSV-1 — the cause of cold sores). The alterations in the virus include incorporating the gene for GM-CSF and a mutation that prevents the virus from infecting non-dividing cells while preserving its ability to infect and replicate in cancer cells. Replication kills the cells and causes them to release:
- more viruses which spread the infection;
- tumor antigens, and
- GM-CSF which attracts dendritic cells to the site. These take up the tumor antigens and present them to T cells that go on to mount an attack against surviving tumor cells.
(Tumor cell death by HSV does not qualify it as immunotherapy, but the T-cell response that results certainly does.)


