15.4: Environmental Toxicology
- Page ID
- 31634
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Environmental toxicology is the scientific study of the properties of toxins, chemicals that may cause damage to living organisms, and the health effects associated exposure to them (table \(\PageIndex{a}\)). The field also involves managing these toxins and the protecting humans and ecosystems from them. Toxicologists are scientists who study the properties of toxins, and these damage-causing properties, are called toxicity. In other words, toxicologists evaluate chemical hazards.
| 2019 Rank | Substance Name |
|---|---|
| 1 | Arsenic |
| 2 | Lead |
| 3 | Mercury |
| 4 | Vinyl chloride |
| 5 | Polychlorinated biphenyls (PCBs) |
| 6 | Benzene |
| 7 | Cadmium |
| 8 | Benzo[a]pyrene |
| 9 | Polycyclic aromatic hydrocarbons |
| 10 |
Benzo[b]fluoranthene |
| 11 | Chloroform |
| 12 |
Aroclor 1260 |
| 13 | p,p'-DDT |
| 14 | Aroclor 1254 |
| 15 | Dibenz[a,h]anthracene |
Table modified from ATSDR/CDC (public domain).
What Forms do Chemicals Take?
Chemical substances can take a variety of forms. They can be in the form of solids, liquids, dusts, vapors, gases, fibers, mists and fumes (figure \(\PageIndex{a}\)). The form a substance is in has a lot to do with how it gets into your body and what harm it can cause. A chemical can also change forms. For example, liquid solvents can evaporate and give off vapors that you can inhale. Sometimes chemicals are in a form that can’t be seen or smelled, so they can’t be easily detected.

Routes of Exposure to Chemicals
In order to cause health problems, chemicals must enter your body. Once chemicals have entered your body, some can move into your bloodstream and reach internal “target” organs, such as the lungs, liver, kidneys, or nervous system. There are three main routes of exposure, or ways a chemical can get into your body (figure \(\PageIndex{b}\)).
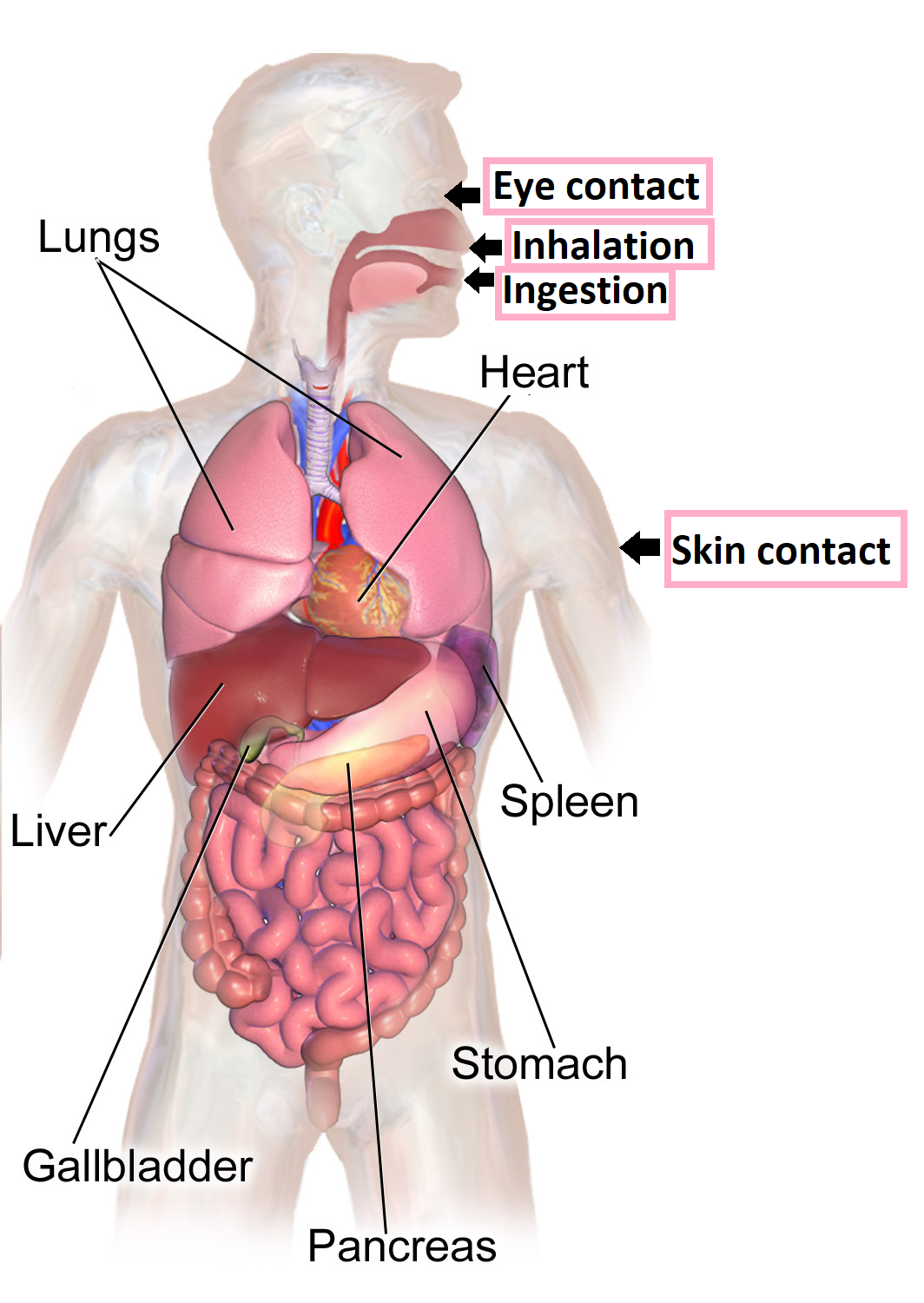
The first route is inhalation, which results from breathing in chemical gases, mists, or dusts that are in the air. Because the air sacs in the lungs are structure for gas exchange, consisting of only a single layer of thin cells, inhaled substances can rapidly pass from the air sacs into capillaries and enter the bloodstream. Inhaled toxins can also cause local damage to the mouth, airways, and lungs.
The second route is ingestion, swallowing that occurs chemicals have spilled or settled onto food, beverages, cigarettes, beards, or hands. Toxins can then cause local damage to the digestive tract and may be absorbed, most often through the intestines, into the blood stream.
The third route is skin or eye contact. When chemicals directly touch the skin or get in the eyes, they can cause localized damage or be absorbed through the skin into the bloodstream. Because the skin consists of many cell layers that are reinforced with protective compounds, it is more difficult for toxins to enter the body through the skin compared to inhalation and ingestion. However, toxins can easily enter through damaged skin, and some substances can be absorbed through intact skin. Because the eyes have a rich blood supply, toxins can ready enter the bloodstream through eye contact.
What Factors Impact a Chemical's Safety?
Why are some chemicals more harmful than other? Many factors are considered when evaluating a chemical's safety including potency, persistence, solubility, bioaccumulation and biomagnification. Potency refers to the amount of a chemical needed to cause harm. The more potent a toxin, the lower the concentration needed to cause harm. Persistence refers to how long a substance takes to break down. Persistent chemicals are of greater concern because they remain in the environment (or even in organisms) for long time periods. Solubility refers to whether the chemical dissolves in certain solvents, such as water or fat. Generally, fat-soluble (lipid-soluble) toxins are more dangerous because they can accumulate in fatty tissues (see below) whereas water-soluble toxins could be more easily flushed out of the body. Furthermore, fat-soluble toxins are more easily absorbed by the body.
Bioaccumulation
Bioaccumulation is the buildup of chemicals in an organism’s tissues over its lifetime. While bioaccumulated toxins are commonly fat soluble, such as DDT and PCBs, water-soluble toxins, such as inorganic forms of heavy metals can also bioaccumulate. For example, lead accumulates in the teeth and bone, and mercury can accumulate in the kidneys and brain.
Biomagnification
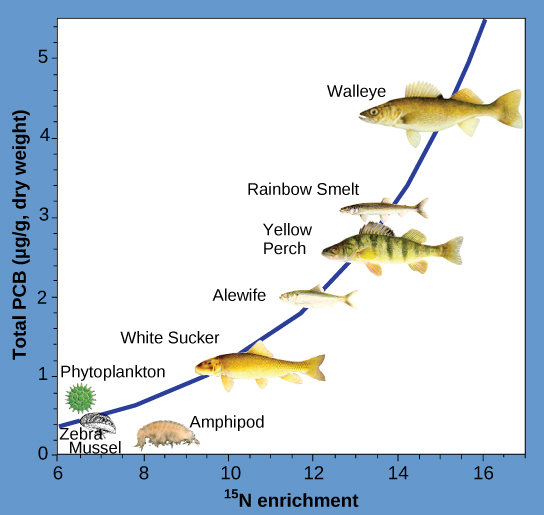
Biomagnification is the increasing concentration of toxins in organisms at each successive trophic level. When organisms with bioaccumulated toxins are consumed, the toxins are transferred to their predators (figure \(\PageIndex{d}\)). Biomagnification explains why some fish species high on the food chain contain high concentrations of mercury and cadmium, another heavy metal.

In addition to high persistence, fat solubility, and bioaccumulation, biomagnification explains why the now-banned insecticide dichlorodiphenyltrichloroethane (DDT) caused so much damage. Producers absorbed DDT and passed it on to successive levels of consumers at increasingly higher rates. For example, spraying a marsh to control mosquitoes will cause trace amounts of DDT to accumulate in the cells of microscopic aquatic organisms, the plankton, in the marsh. In feeding on the plankton, filter-feeders, like clams and some fish, harvest DDT as well as food. (Concentrations of DDT 10 times greater than those in the plankton have been measured in clams.) The process of concentration goes right on up the food chain from one trophic level to the next. Gulls, which feed on clams, may accumulate DDT to 40 or more times the concentration in their prey. This represents a 400-fold increase in concentration along the length of this short food chain. Ultimately, apex predators at the top of the food chain such as Bald Eagles, pelicans, falcons, and ospreys fed on contaminated fish, reaching dangerous DDT levels.
Another substances that biomagnifies is polychlorinated biphenyl (PCB). The National Atmospheric and Oceanic Administration (NOAA) studied biomagnification of PCB in the Saginaw Bay of Lake Huron of the North American Great Lakes (figure \(\PageIndex{d}\)). Concentrations of PCB increased from the producers of the ecosystem (phytoplankton) through the different trophic levels of fish species. The apex predator, the walleye, had more than four times the amount of PCBs compared to phytoplankton. Also, research found that birds that eat these fish may have PCB levels that are at least ten times higher than those found in the lake fish. This aquatic ecosystem offered an ideal opportunity to study biomagnification because PCB generally exists at low concentrations in this environment, but apex predators accumulated very high concentrations of the toxin.
Persistent Organic Pollutants (POPs)
Persistent organic pollutants (POPs) are a group of organic chemicals that pose risks to human health and ecosystems. Examples include the pesticide dichlorodiphenyltrichloroethane (DDT) and the industrial chemicals polychlorinated biphenyls (PCBs) and per- and polyfluoroalkyl substances (PFAS). The contaminant in Agent Orange (2,3,7,8-tetrachlorodibenzo-p-dioxin; TCDD) is another POP (see The Scientific Method). Persistent organic pollutants have the following three characteristics:
- Persistent: POPs are chemicals that last a long time in the environment. Some may resist breakdown for years and even decades while others could potentially break down into other toxic substances.
- Bioaccumulative: POPs can accumulate in animals and humans, usually in fatty tissues and largely from the food they consume. As these compounds move up the food chain, they concentrate to levels that could be thousands of times higher than acceptable limits.
- Toxic: POPs can cause a wide range of health effects in humans, wildlife and fish. They have been linked to effects on the nervous system, reproductive and developmental problems, suppression of the immune system, cancer, and endocrine disruption. The deliberate production and use of most POPs has been banned around the world, with some exemptions made for human health considerations (for example, DDT for malaria control) and/or in very specific cases where alternative chemicals have not been identified. However, the unintended production and/or the current use of some POPs continue to be an issue of global concern. Even though most POPs have not been manufactured or used for decades, they continue to be present in the environment and thus potentially harmful. The same properties that originally made them so effective, particularly their stability, make them difficult to eradicate from the environment.
The relationship between exposure to environmental contaminants such as POPs and human health is complex. There is mounting evidence that these persistent, bioaccumulative and toxic chemicals (PBTs) cause long-term harm to human health and the environment. Drawing a direct link, however, between exposure to these chemicals and health effects is complicated, particularly since humans are exposed on a daily basis to many different environmental contaminants through the air they breathe, the water they drink, and the food they eat. Numerous studies link POPs with a number of adverse effects in humans. These include effects on the nervous system, problems related to reproduction and development, cancer, and genetic impacts. Moreover, there is mounting public concern over the environmental contaminants that mimic hormones in the human body (endocrine disruptors).
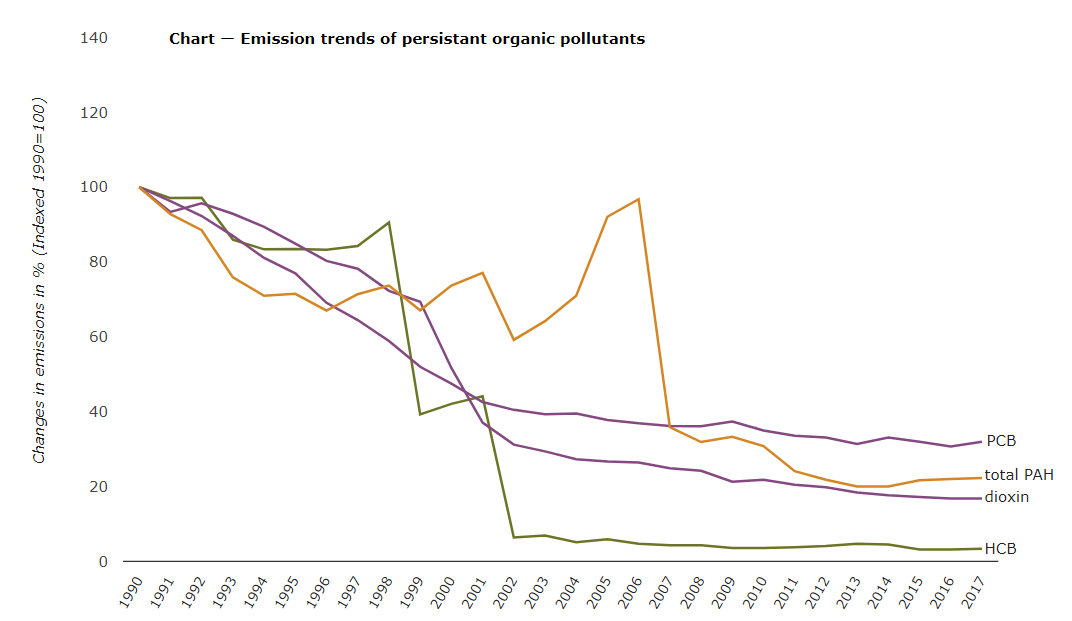
Through atmospheric processes, they are deposited onto land or into water ecosystems where they accumulate and potentially cause damage. From these ecosystems, they evaporate, again entering the atmosphere, typically traveling from warmer temperatures toward cooler regions. They condense out of the atmosphere whenever the temperature drops, eventually reaching highest concentrations in circumpolar countries. Through these processes, POPs can move thousands of kilometers from their original source of release in a cycle that may last decades.
As with humans, animals are exposed to POPs in the environment through air, water and food. POPs can remain in sediments for years, where bottom-dwelling creatures consume them and who are then eaten by larger fish. Because tissue concentrations can biomagnify at each level of the food chain, top predators, including whales, seals, polar bears, birds of prey, tuna, swordfish and bass may have a million times greater concentrations of POPs than the water itself. Once POPs are released into the environment, they may be transported within a specific region and across international boundaries transferring among air, water, and land.
While generally banned or restricted (figure \(\PageIndex{e}\)), POPs make their way into and throughout the environment on a daily basis through a cycle of long-range air transport and deposition called the “grasshopper effect.”The “grasshopper” processes begin with the release of POPs into the environment. When POPs enter the atmosphere, they can be carried with wind currents, sometimes for long distances.
What Are the Health Effects of Toxins?
A toxin's effect is determined by many factors. Firstly, the same toxin and the same concentration can affect individuals differently depending on age, overall health, genetics, gender, and other factors. For example, young children are especially susceptible to heavy metals and bisphenol A (BPA). Additionally, as many toxins are processed by the liver, and liver function decreases with age, elderly individuals are more susceptible to certain toxins. Similarly, an generally health individual is likely to withstand exposure toxins better than someone facing other health concerns. Regarding genetics, some individuals may have versions of genes that make them more susceptible or resistant to certain toxins. In addition to individual factors, the length of exposure, presence of other toxins, and concentration (dosage) all impact toxicity
Acute vs. Chronic Effects
An acute effect of a toxin is one that occurs rapidly after exposure to a large amount of that substance. A chronic effect of a contaminant results from exposure to small amounts of a substance over a long period of time. In such a case, the effect may not be immediately obvious. Chronic effects are difficult to measure, as the effects may not be seen for years. Long-term exposure to cigarette smoking, low-level radiation exposure, and moderate alcohol use are all thought to produce chronic effects.
Toxicological Interactions
Exposure to multiple chemicals simultaneously can result in a variety of effects, or chemical interactions (figure \(\PageIndex{f}\)). These can apply to any chemicals that affect the body, including drugs and toxins (in the latter case, they are called toxicological interactions.) If the effect of the two chemicals combined is the sum of their individual effects, the chemical interaction is called an additive effect. Suppose that drug A and drug B have the same effect on the body (for example, each increasing heart rate by five beats per minute). An additive effect would occur if taking both drugs together increased the heart rate by 10 beats per minute. For example, toluene and xylene, which are both found in solvents and paints have an additive effect. Each causes eye, nose, and throat irritation, dizziness, headaches, and confusion. A study found that their combined effects on memory, cognitive function, and coordination was roughly equal to the sum of their individual effects.
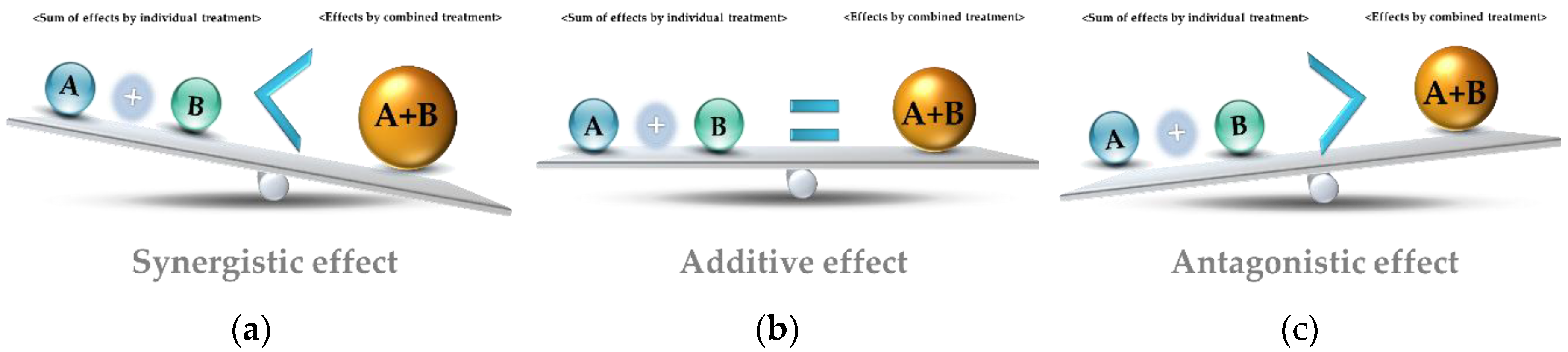
In contrast, if taking both drugs A and B simultaneously increased heart rate by less than 10 beats per minute (less than their sum), their interaction would be considered an antagonistic effect, indicating that they interfere with each other. For example, ethanol (found in alcoholic beverages) and methanol (wood alcohol) both have toxic effects, but methanol is more immediately damaging. When methanol is ingested, they body converts it to dangerous compounds that cannot be easily removed from the body. Ethanol can used to treat methanol poisoning because it blocks the enzymes that facilitate these reactions, providing the body an opportunity to eliminate the methanol.
If combining drugs A and B increased heart rate more than 10 beats per minute, their interaction would be a synergistic effect; that is, their combined effect was greater than the sum of their individual effects. For example, smoking combined with asbestos exposure have a synergistic effect in causing lung cancer.
The Dose-Response Curve
For centuries, scientists have known that just about any substance is toxic in sufficient quantities. It is a thus common saying among toxicologists that "the dose makes the poison". In fact, too high a dosage (amount) of an otherwise helpful drug can cause negative health effects or even death. Similarly, small amounts of selenium are required by living organisms for proper functioning, but large amounts may cause cancer.
The effect of a certain chemical on an individual depends on the dose of the chemical. This relationship is often illustrated by a dose-response curve, which shows the relationship between dose and the response of the individual. Lethal doses in humans have been determined for many substances from information gathered from records of homicides, accidental poisonings, testing on animals, and experiments on cell cultures. A dose that is lethal to 50% of a population of test animals is called the lethal dose-50%, or LD50 (Table \(\PageIndex{b}\)). Determination of the LD50 is required for new synthetic chemicals in order to give a measure of their toxicity. Because a single conventional LD50 test may kill as many as 100 animals, the United States and other members of the Organization for Economic Cooperation and Development agreed in December 2000 to phase out the LD50 test in favor of alternatives that greatly reduce (or even eliminate) deaths of the test animals.
(mg/kg)
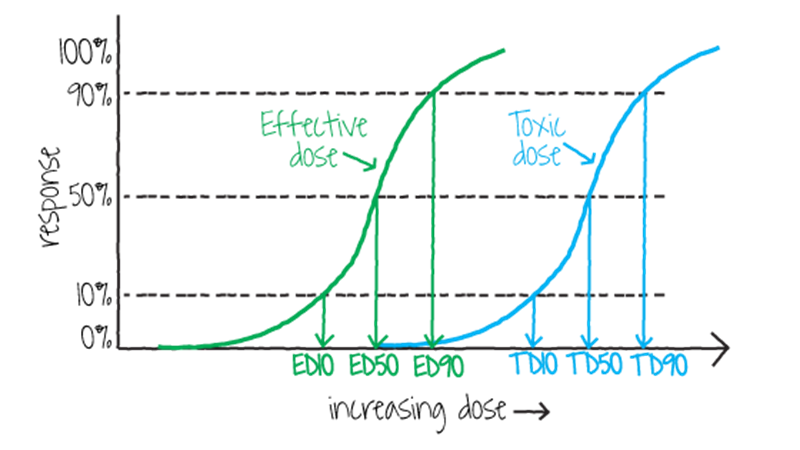
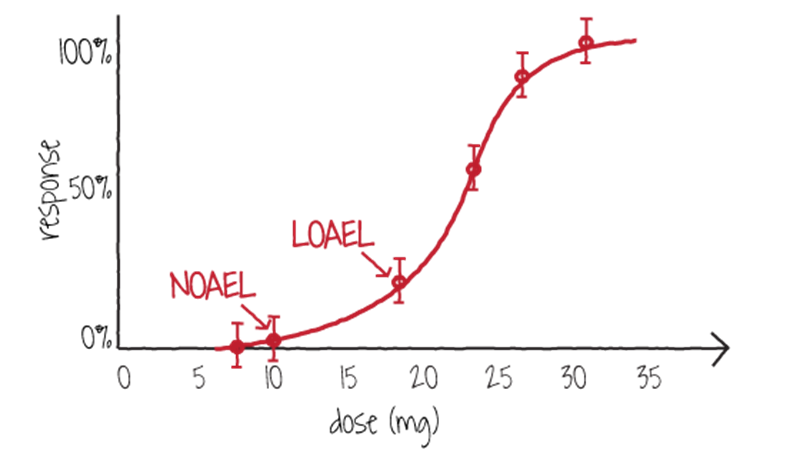
A dose that causes 50% of a population to exhibit any significant response, whether therapeutic or harmful (hair loss, stunted development, etc.), is referred to as the effective dose-50%, or ED50 (figure \(\PageIndex{h}\)). Some toxins have a threshold dose below which there is no apparent effect on the exposed population, called the no-observed-adverse-effect level (NOAEL; figure \(\PageIndex{i}\)). The lowest dose at which any negative effect is apparent is called the lowest-observed-adverse-effect level (LOAEL). Between NOAEL and LOAEL, there may be a noticeable but harmless effect.
Human Health, Environmental, and Economic Effects of Pesticide Use in Potato Production in Ecuador
The International Potato Center (CIP) conducted an interdisciplinary and inter-institutional research intervention project dealing with pesticide impacts on agricultural production, human health, and the environment in Carchi, Ecuador. Carchi is the most important potato-growing area in Ecuador, where smallholder farmers dominate production. They use tremendous amounts of pesticides for the control of the Andean potato weevil and the late blight fungus. Virtually all farmers apply highly hazardous pesticides (classified by the World Health Organization as class Ib) using hand pump backpack sprayers (figure \(\PageIndex{g}\)). The LD50 for class Ib pesticides is 5-50 or 20-200 mg/kg for oral ingestion in solid or liquid form, respectively. For dermal (skin) exposure, the LD50 is 10-100 or 40-400 mg/kg for solids and liquids, respectively. The LD50 class III pesticides, which are considered only slightly hazardous, is at least 10 times greater that that for class Ib pesticides.

The study found that the health problems caused by pesticides are severe and are affecting a high percentage of the rural population. Despite the existence of technology and policy solutions, government policies continue to promote the use of pesticides. The study conclusions concurred with those by the pesticide industry, “that any company that could not ensure the safe use of highly toxic pesticides should remove them from the market and that it is almost impossible to achieve safe use of highly toxic pesticides among small farmers in developing countries.”
Source: Yanggen et al. 2003.
Precautionary Principle
Determining a safe dosage from a dose-response curve employs the precautionary principle, which put simply, embodies the phrase “It’s better safe than sorry.” Actions that follow the precautionary principle allow margin to ensure safety in the case that a toxin or drug is later found to have negative effect at a lower dosage than first detected. The safe dosage is often set to 1% or even 0.1% of the NOAEL.
The precautionary principle is sometimes applied to other components of environmental toxicology. For example in the European Union (EU), manufacturers must demonstrate the safety of their product before it is sold. While this is also required in the U.S. for chemicals that have not been used before, there is no such rule for existing products. It is instead the responsibility of Environmental Protection Agency to demonstrate that products already on the market are unsafe before banning them. In summary, the precautionary principle is applied to regulating potential toxins in products in both the EU and U.S.; however, the EU's applies the precautionary principle more broadly. The EU errors on the side of caution, potentially banning chemicals that are harmless until they are proven safe, but the U.S. risks exposure to potential toxins before their safety is determined.
Attribution
Modified by Melissa Ha from the following sources:
- Persistent Organic Pollutants: Backyards to Borders. 2004. World Bank. (licensed under CC-BY)
- Environmental Biology by Matthew R. Fisher (licensed under CC-BY)
- Biomagnification, Insecticides, and the LD50 test from Biology by John W. Kimball (licensed under CC-BY)


