5.5B: The Ability to Resist Phagocytic Engulfment (Attachment and Ingestion) and Antibacterial Peptides
- Page ID
- 3166
- Briefly describe at least 3 ways capsules may enable bacteria to resist phagocytic engulfment and state how this can promote colonization.
- State at least 2 mechanisms other than capsules that certain bacteria might use to resist phagocytic engulfment.
- State 3 ways bacteria might resist antibacterial peptides like defensins.
Highlighted Bacterium
- Read the description of Haemophilus influenzae and match the bacterium with the description of the organism and the infection it causes.
We will now look virulence factors that enable bacteria to resist phagocytic engulfment (attachment and ingestion) and antibacterial peptides. As we learned in Unit 1, capsule enable many organisms to resist phagocytic engulfment. For example, Streptococcus pneumoniae is able to initially evade phagocytosis and cause infections such as pneumococcal pneumonia, sinusitis, otitis media, and meningitis because of its capsule. Encapsulated strains of Haemophilus influenzae type b can causes severe respiratory infections, septicemia, epiglottitis, and meningitis in children (other non-encapsulated strains of H. influenzae usually cause mild respiratory infections such as sinusitis and otitis media). Other encapsulated bacteria include Neisseria meningitidis, Bacillus anthracis, and Bordetella pertussis.
Click on this link, read the description of Haemophilus influenzae, and be able to match the bacterium with its description on an exam.
Capsules that resist Unenhanced Attachment
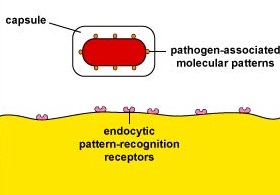
Capsules can resist unenhanced attachment by preventing pathogen-associated molecular patterns or PAMPs - components of common molecules such as peptidoglycan, teichoic acids, lipopolysaccharide, mannans, and glucans common in microbial cell walls but not found on human cells - from binding to endocytic pattern-recognition receptors on the surface of the phagocytes (Figure \(\PageIndex{1}\)).
Capsules that Interfere with Complement Pathways
The capsules of some bacteria interfere with the host's complement pathways and do so in a number of ways:
The capsules of some bacteria prevent the formation of C3 convertase, an early enzyme in the complement pathways. Without this enzyme, the opsonins C3b and C4b involved in enhanced attachment, as well as the other beneficial complement proteins like C5a, are not produced.
Some capsules are rich in sialic acid, a common component of host cell glycoprotein. Sialic acid has an affinity for serum protein H, a complement regulatory protein that leads to the degradation of the opsonin C3b and the formation of C3 convertase. (Our body uses serum protein H to degrade any C3b that binds to host cell glycoproteins so that we don't stick our own phagocytes to our own cells with C3b.) Some Neisseria meningitidis strains synthesize their own sialic acid capsule. While Neisseria gonorrhoeae and Hemophilus influenzae type b do not have a sialic acid capsule, they are able to scavenge sialic acid from host cells and enzymatically transfer it to their surface where it subsequently binds protein H.
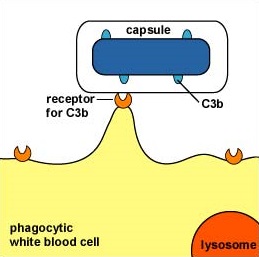
Some capsules simply cover the C3b that does bind to the bacterial surface and prevent the C3b receptor on phagocytes from making contact with the C3b (Figure \(\PageIndex{2}\)). This is seen with the capsule of Streptococcus pneumoniae.
Staphylococcus aureus produces a protein called Staphylococcal complement inhibitor that binds and inhibits the C3 convertase enzyme needed for all three complement pathways.
The body's immune defenses, however, can eventually get around these capsule by producing opsonizing antibodies (IgG) that stick capsules to the phagocyte. In vaccines against pneumococccal pneumonia and Haemophilus influenzae type b, it is capsular polysaccharide that is given as the antigen to stimulate the body to make opsonizing antibodies against the encapsulated bacterium.
Biofilms
Many pathogenic bacteria, as well as normal flora, form complex bacterial communities as biofilms. Bacteria in biofilms are often able to communicate with one another by a process called quorum sensing and are able to interact with and adapt to their environment as a population of bacteria rather than as individual bacteria. By living as a community of bacteria as a biofilm, these bacteria are better able to:
- resist attack by antibiotics;
- trap nutrients for bacterial growth and remain in a favorable niche;
- adhere to environmental surfaces and resist flushing;
- live in close association and communicate with other bacteria in the biofilm; and
- resist phagocytosis and attack by the body's complement pathways.
Biofilms are, therefore, functional, interacting, and growing bacterial communities. Biofilms even contain their own water channels for delivering water and nutrients throughout the biofilm community. For example, Pseudomonas aeruginosa produces a glycocalyx composed of alginate. This enables strains producing the glycocalyx to block neutrophil chemotaxis, scavenge the hypochlorite molecules produced by neutrophils to kill bacteria, decrease phagocytosis, and inhibit activation of the complement pathways.
Other Mechanisms
The M-protein of Streptococcus pyogenes allows these bacteria to be more resistant to phagocytic engulfment. The M-protein of S. pyogenes binds factor H, a complement regulatory protein that leads to the degradation of the opsonin C3b and the formation of C3 convertase. (Our body uses serum protein H to degrade any C3b that binds to host cell glycoproteins so that we don't stick our own phagocytes to our own cells with C3b.) S. pyogenes also produces a protease that cleaves the complement protein C5a.
Coagulase, produced by Staphylococcus aureus. Coagulase causes fibrin clots to form around the organism that help enable it to resist phagocytosis. Our adaptive immune system has difficulty in recognizing the S. aureus as foreign when it is coated with a human protein.
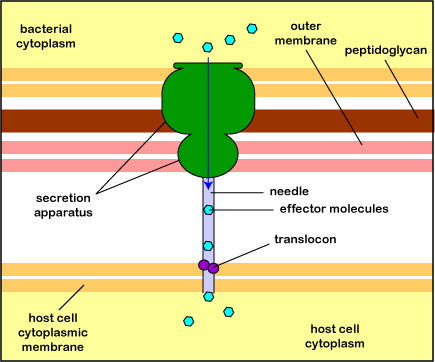
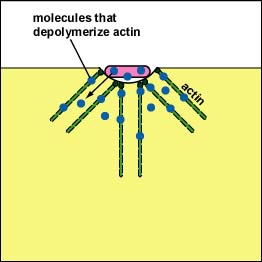
Pathogenic Yersinia, such as the species that causes plague, Y. pestis, contact phagocytes and, by means of a type III secretion system (Figure \(\PageIndex{3}\)), deliver proteins that depolymerize the actin microfilaments needed for phagocytic engulfment into the phagocytes (Figure \(\PageIndex{4}\)).
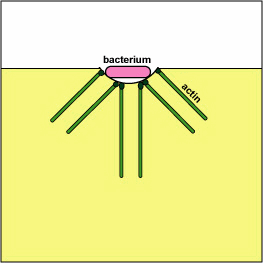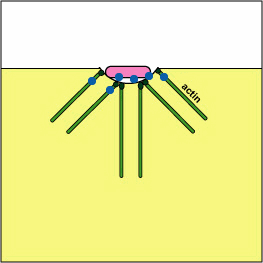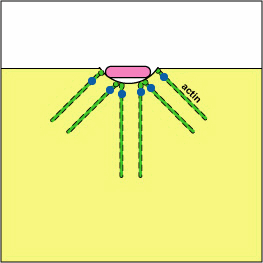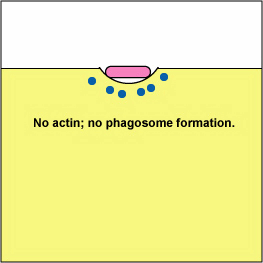
Blocking Phagosome Formation by Depolymerizing Actin. Molecules of some bacteria, through a type III secretion system, deliver proteins that depolymerize the phagocyte's actin microfilaments used for phagocytic engulfment.
The pili (fimbriae) of Streptococcus pyogenes both blocks the activation of the complement pathways on the bacterial cell wall and helps to resist phagocytic engulfment.
The vaccine for Haemophilus influenzae type b contains capsular material from this bacterium. The body recognizes this capsular material as foreign and produces antibodies against it. Describe how this might this protect the person from infection with this bacterium compared to a person who is not immunized.
Certain bacteria can resist antibacterial peptides
Human defensins are short cationic peptides 29-34 amino acids long that are directly toxic by forming pores in the cytoplasmic membrane of a variety of microorganisms causing leakage of cellular needs. They also activate cells for an inflammatory response. Defensins are produced by leukocytes, epithelial cells, and other cells. They are also found in blood plasma and mucus. Cathelicidinsare proteins produced by skin and mucosal epithelial cells. The two peptides produced upon cleavage of the cathelicidin are directly toxic to a variety of microorganisms. One pepitide also can bind to and neutralize LPS from Gram-negative cell walls to reduce inflammation.
- Capsules help prevent antibacterial peptides from reaching the cytoplasmic membrane of some bacteria.
- The lipopolysaccharide (LPS) of the gram-negative cell wall binds cationic antibacterial peptides and prevents them from reaching the cytoplasmic membrane.
- Some bacteria secrete peptidases that break down antibacterial peptides.
Summary
- Capsules can resist unenhanced attachment by by preventing pathogen-associated molecular patterns or from binding to endocytic pattern-recognition receptors on the surface of the phagocytes.
- The capsules of some bacteria interfere with the body's complement pathway defenses.
- The body's immune defenses can eventually get around the capsule by producing opsonizing antibodies (IgG) against the capsule that stick the capsule to the phagocyte. This is the principle behind some vaccines.
- Biofilms enable bacteria to: resist attack by antibiotics; trap nutrients for bacterial growth and remain in a favorable niche; adhere to environmental surfaces and resist flushing; live in close association and communicate with other bacteria in the biofilm; and resist phagocytosis and attack by the body's complement pathways.
- Certain bacteria can resist antibacterial peptides.


