7.2: Examples of Endospores
- Page ID
- 123365
a. Anthrax, caused by Bacillus anthracis. (See Fig. \(\PageIndex{5A}\) and Fig. \(\PageIndex{1B}\).)
Endospores can be inhaled, ingested, or enter wounds where they germinate and the vegetative bacteria subsequently replicate and produce exotoxins. In the case of the two anthrax exotoxins, two different A-components known as lethal factor (LF) and edema factor (EF) share a common B-component known as protective antigen (PA). Protective antigen, the B-component, first binds to receptors on host cells and is cleaved by a protease creating a binding site for either lethal factor or edema factor. At low levels, LF inhibits the release of pro-inflammatory cytokines such as interleukin-1 (IL-1), tumor necrosis factor-alpha, (TNF-alpha), and NO. This may initially reduce immune responses against the organism and its toxins. But at high levels, LF is cytolytic for macrophages, causing release of high levels of interleukin-1 (IL-1), tumor necrosis factor-alpha (TNF-alpha), and NO. Excessive release of these cytokines can lead to a massive inflammatory response and the shock cascade, similar to septic shock. Edema factor impairs phagocytosis, and inhibits production of TNF and interleukin-6 (IL-6) by monocytes. This most likely impairs host defenses.
|
Fig. \(\PageIndex{1A}\): Endospore stain of Bacillus anthracis |
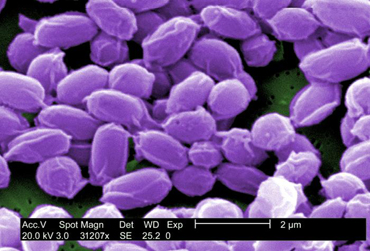
Fig. \(\PageIndex{1B}\): Scanning Electron Micrograph of the Endospores of Bacillus anthracis |
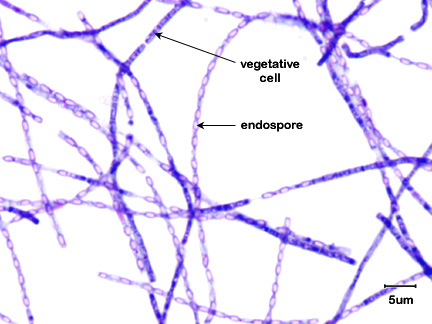 Note the endospores within the streptobacillus. Note the endospores within the streptobacillus. |
 |
|
Gary E. Kaiser, Ph.D. |
Image provided by Janice Haney Carr. Courtesy of the Centers for Disease Control and Prevention. |
b. Tetanus, caused by Clostridium tetani. (See Fig. \(\PageIndex{2}\).)
Endospores enter anaerobic wounds where they germinate and the vegetative bacteria subsequently replicate and release exotoxin. Tetanus exotoxin (tetanospasmin), produced by Clostridium tetani is a neurotoxin that binds to inhibitory interneurons of the spinal cord and blocks their release of inhibitor molecules. It is these inhibitor molecules from the inhibitory interneurons that eventually allow contracted muscles to relax by stopping excitatory neurons from releasing the acetylcholine that is responsible for muscle contraction. The toxin, by blocking the release of inhibitors, keeps the involved muscles in a state of contraction and leads to spastic paralysis, a condition where opposing flexor and extensor muscles simultaneously contract. Death is usually from respiratory failure.
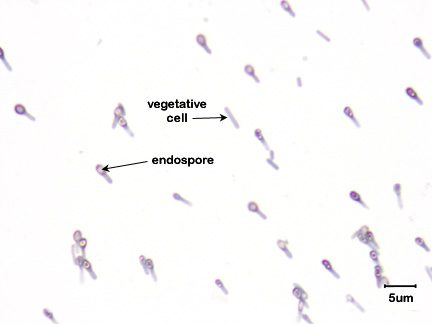
c. Botulism, caused by Clostridium botulinum. (See Fig. \(\PageIndex{3}\))
Endospores enter the anaerobic environment of improperly canned food where they germinate and subsequently replicate and at a neutral pH, secrete botulinal exotoxin. This is a neurotoxin that acts peripherally on the autonomic nervous system. For muscle stimulation, acetylcholine must be released from the neural motor end plate of the neuron at the synapse between the neuron and the muscle to be stimulated. The acetylcholine then induces contraction of the muscle fibers. The botulism exotoxin binds to and enters the presynaptic neuron and blocks its release of acetylcholine. This causes a flaccid paralysis, a weakening of the involved muscles. Death is usually from respiratory failure.
d. Gas gangrene, caused by Clostridium perfringens. (See Fig. \(\PageIndex{4}\))
Endospores enter anaerobic wounds where they germinate and the vegetative bacteria subsequently replicate and produce a variety of exotoxins. This bacterium produces at least 20 exotoxins that play a role in the pathogenesis of gas gangrene and producing expanding zones of dead tissue (necrosis) surrounding the bacteria. Toxins include: Alpha toxin (lecithinase) that increases the permeability of capillaries and muscle cells by breaking down lecithin in cytoplasmic membranes resulting in the gross edema associated with gas gangrene as well as being necrotizing, hemolytic, and cardiotoxic; Kappa toxin (collagenase) breaks down supportive connective tissue resulting in the mushy lesions of gas gangrene and is also necrotizing; Mu toxin (hyaluronidase) breaks down the tissue cement that holds cells together in tissue; and epsilon toxin Increases vascular permeability and causes edema and congestion in various organs including lungs and kidneys. Additional necrotizing toxins include beta toxin, iota toxin, and nu toxin. A major characteristic of gas gangrene is the ability of C. perfringens to very rapidly spread from the initial wound site, leaving behind an expanding zone of dead tissue. This organism spreads as a result of the pressure from fluid accumulation (due to increased capillary permeability from alpha toxin) and gas production (anaerobic fermentation of glucose by the organisms produces hydrogen and carbon dioxide), coupled with the breakdown of surrounding connective tissue (kappa toxin) and tissue cement (mu toxin).
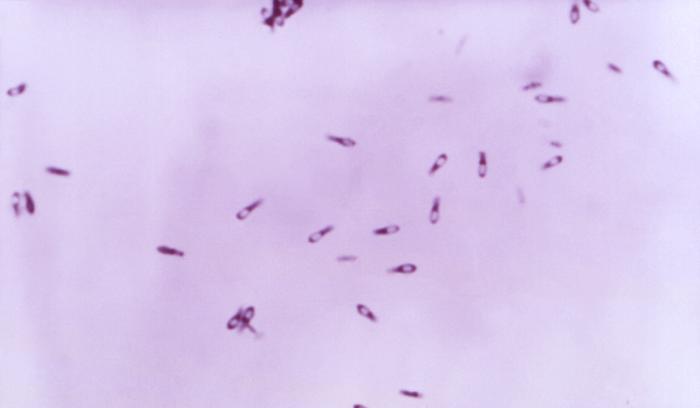
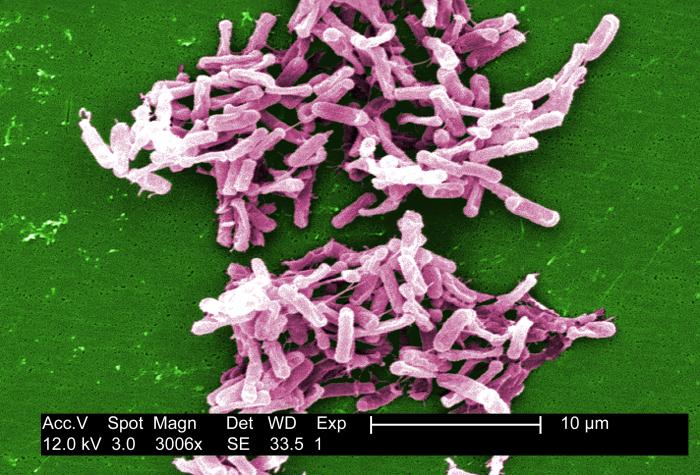
e. Antibiotic-associated pseudomembranous colitis, caused by Clostridioides difficile, formerly known as Clostridium difficile. (See Fig. \(\PageIndex{5}\))
Clostridioides difficile causes severe antibiotic-associated colitis and is an opportunistic Gram-positive, endospore-producing bacillus transmitted by the fecal-oral route. C. difficile is a common health-care-associated infection (HAIs) and is the most frequent cause of health-care-associated diarrhea. C. difficile infection often recurs and can progress to sepsis and death. CDC has estimated that there are about 500,000 C. difficile infections (CDI) in health-care associated patients each year and is linked to 15,000 American deaths each year. Antibiotic-associated colitis is especially common in older adults. It is thought that C. difficile survives the exposure to the antibiotic by sporulation. After the antibiotic is no longer in the body, the endospores germinate and C. difficile overgrows the intestinal tract and secretes toxin A and toxin B that have a cytotoxic effect on the epithelial cells of the colon. C. difficile has become increasingly resistant to antibiotics in recent years making treatment often difficult. There has been a great deal of success in treating the infection with fecal transplants.
 |
Due to the resistant nature of the endospore coats, endospores are difficult to stain. Strong dyes and vigorous staining conditions such as heat are needed. Once stained, however, endospores are equally hard to decolorize. Since few bacterial genera produce endospores, the endospore stain is a good diagnostic test for species of Bacillus and Clostridium.
Contributors and Attributions
Dr. Gary Kaiser (COMMUNITY COLLEGE OF BALTIMORE COUNTY, CATONSVILLE CAMPUS)

