2.8: Procedure
\newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} }
\newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}}
\newcommand{\id}{\mathrm{id}} \newcommand{\Span}{\mathrm{span}}
( \newcommand{\kernel}{\mathrm{null}\,}\) \newcommand{\range}{\mathrm{range}\,}
\newcommand{\RealPart}{\mathrm{Re}} \newcommand{\ImaginaryPart}{\mathrm{Im}}
\newcommand{\Argument}{\mathrm{Arg}} \newcommand{\norm}[1]{\| #1 \|}
\newcommand{\inner}[2]{\langle #1, #2 \rangle}
\newcommand{\Span}{\mathrm{span}}
\newcommand{\id}{\mathrm{id}}
\newcommand{\Span}{\mathrm{span}}
\newcommand{\kernel}{\mathrm{null}\,}
\newcommand{\range}{\mathrm{range}\,}
\newcommand{\RealPart}{\mathrm{Re}}
\newcommand{\ImaginaryPart}{\mathrm{Im}}
\newcommand{\Argument}{\mathrm{Arg}}
\newcommand{\norm}[1]{\| #1 \|}
\newcommand{\inner}[2]{\langle #1, #2 \rangle}
\newcommand{\Span}{\mathrm{span}} \newcommand{\AA}{\unicode[.8,0]{x212B}}
\newcommand{\vectorA}[1]{\vec{#1}} % arrow
\newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow
\newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} }
\newcommand{\vectorC}[1]{\textbf{#1}}
\newcommand{\vectorD}[1]{\overrightarrow{#1}}
\newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}}
\newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}}
\newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} }
\newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}}
\newcommand{\avec}{\mathbf a} \newcommand{\bvec}{\mathbf b} \newcommand{\cvec}{\mathbf c} \newcommand{\dvec}{\mathbf d} \newcommand{\dtil}{\widetilde{\mathbf d}} \newcommand{\evec}{\mathbf e} \newcommand{\fvec}{\mathbf f} \newcommand{\nvec}{\mathbf n} \newcommand{\pvec}{\mathbf p} \newcommand{\qvec}{\mathbf q} \newcommand{\svec}{\mathbf s} \newcommand{\tvec}{\mathbf t} \newcommand{\uvec}{\mathbf u} \newcommand{\vvec}{\mathbf v} \newcommand{\wvec}{\mathbf w} \newcommand{\xvec}{\mathbf x} \newcommand{\yvec}{\mathbf y} \newcommand{\zvec}{\mathbf z} \newcommand{\rvec}{\mathbf r} \newcommand{\mvec}{\mathbf m} \newcommand{\zerovec}{\mathbf 0} \newcommand{\onevec}{\mathbf 1} \newcommand{\real}{\mathbb R} \newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]} \newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]} \newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]} \newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]} \newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]} \newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]} \newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]} \newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]} \newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]} \newcommand{\laspan}[1]{\text{Span}\{#1\}} \newcommand{\bcal}{\cal B} \newcommand{\ccal}{\cal C} \newcommand{\scal}{\cal S} \newcommand{\wcal}{\cal W} \newcommand{\ecal}{\cal E} \newcommand{\coords}[2]{\left\{#1\right\}_{#2}} \newcommand{\gray}[1]{\color{gray}{#1}} \newcommand{\lgray}[1]{\color{lightgray}{#1}} \newcommand{\rank}{\operatorname{rank}} \newcommand{\row}{\text{Row}} \newcommand{\col}{\text{Col}} \renewcommand{\row}{\text{Row}} \newcommand{\nul}{\text{Nul}} \newcommand{\var}{\text{Var}} \newcommand{\corr}{\text{corr}} \newcommand{\len}[1]{\left|#1\right|} \newcommand{\bbar}{\overline{\bvec}} \newcommand{\bhat}{\widehat{\bvec}} \newcommand{\bperp}{\bvec^\perp} \newcommand{\xhat}{\widehat{\xvec}} \newcommand{\vhat}{\widehat{\vvec}} \newcommand{\uhat}{\widehat{\uvec}} \newcommand{\what}{\widehat{\wvec}} \newcommand{\Sighat}{\widehat{\Sigma}} \newcommand{\lt}{<} \newcommand{\gt}{>} \newcommand{\amp}{&} \definecolor{fillinmathshade}{gray}{0.9}MEDIA
- Trypticase Soy Broth tubes (4),
- Trypticase Soy Agar slant tubes (4),
- Trypticase Soy Agar stab tubes (4), and
- Trypticase Soy Agar plates (7).
ORGANISMS
- Trypticase Soy Broth cultures of
- Bacillus subtilis,
- Escherichia coli and
- Micrococcus luteus, and
- Trypticase Soy Agar plate cultures of Mycobacterium phlei.
PROCEDURE (to be done in pairs)
1. Aseptically inoculate one Trypticase Soy Broth tube, one Trypticase Soy Agar slant tube, one Trypticase Soy Agar stab tube, and one Trypticase Soy Agar plate with B. subtilis. (See Fig. \PageIndex{1})
Remember to label all tubes with a wax marker. When streaking the agar plates, use either pattern with three or the pattern with five sectors. This procedure is termed streaking for isolation and has a diluting effect. The friction of the loop against the agar causes organisms to fall off the loop. Near the end of the streaking pattern, individual organisms become separated far enough apart on the agar surface to give rise to isolated single colonies after incubation
2. Aseptically inoculate one Trypticase Soy Broth tube, one Trypticase Soy Agar slant tube, one Trypticase Soy Agar stab tube, and one Trypticase Soy Agar plate with E. coli. (See Fig. \PageIndex{1})
3. Aseptically inoculate one Trypticase Soy Broth tube, one Trypticase Soy Agar slant tube, one Trypticase Soy Agar stab tube, and one Trypticase Soy Agar plate with M. luteus. (See Fig. \PageIndex{1})
4. Aseptically inoculate one Trypticase Soy Broth tube, one Trypticase Soy Agar slant tube, one Trypticase Soy Agar stab tube, and one Trypticase Soy Agar plate with M. phlei. (See Fig. \PageIndex{1})
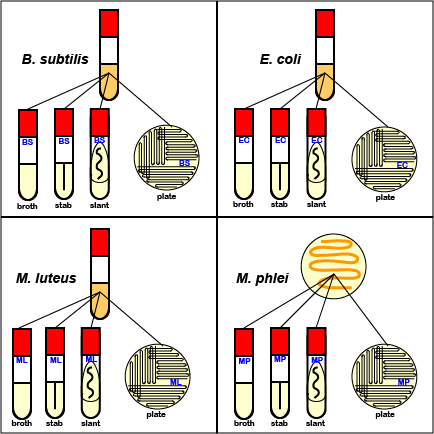
5. Incubate all the tubes and plates inoculated with B. subtilis, E. coli, M. luteus, and M. phlei at 37°C. Place the tubes in your dedidated test tube rack. Incubate the petri plates upside down (lid on the bottom) and stacked in the petri plate holder on the shelf of the 37°C incubator corresponding to your lab section. Incubating the plates upside down prevents condensing water from falling down on the growing colonies and causing them to run together. (Store your test tube rack on your incubator shelf when not in use.)
6. In order to illustrate that microorganisms are all around us and to demonstrate the necessity for proper aseptic technique, contaminate three Trypticase Soy Agar plates as follows:
a. Remove the lid from the first agar plate and place the exposed agar portion in or out of the building for the duration of today's lab. Replace the lid, label the plate "air", and incubate it upside-down at room temperature. Do this plate first.
b. Using a wax marker, divide a second petri plate in half. You and your partner both moisten a sterile cotton swab in sterile water. Rub your swab over some surface in the building or on yourself. Use this swab to inoculate your half of the second agar plate. Label the plate and incubate upside-down at room temperature.
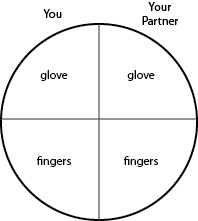
c. With a wax marker, divide a third petri plate into quartersand label as shown in Fig. 13. On your half of the plate, first rub the fingers of one of your gloved hands over your "glove" quadrant. Remove that glove and rub your fingers over your "fingers" quadrant. Your partner will do the same on his or her half of the plate. Label the plate and incubate upside-down in your petri plate holder at 37°C. Do this plate last.
|
Fig. \PageIndex{2A}: Microorganisms growing on an agar plate exposed to the air for one hour. |
Fig. \PageIndex{2B}: An agar plate showing microorganisms growing from the bottom of a shoe. |
Fig. \PageIndex{2C}: Inoculation of Your "Fingers" Plate |
|---|---|---|
 |
||
| (Copyright; Gary E. Kaiser, Ph.D. The Community College of Baltimore County, Catonsville Campus CC-BY-3.0) | ||
Contributors and Attributions
Dr. Gary Kaiser (COMMUNITY COLLEGE OF BALTIMORE COUNTY, CATONSVILLE CAMPUS)


