2.5: Transferring the Inoculum into a Petri Plate
( \newcommand{\kernel}{\mathrm{null}\,}\)
1. If the agar surface of the plate is visibly wet, use a sterile swab to gently remove the water.
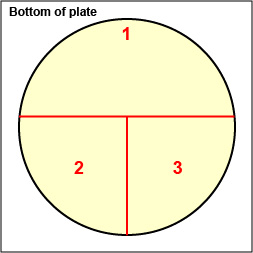
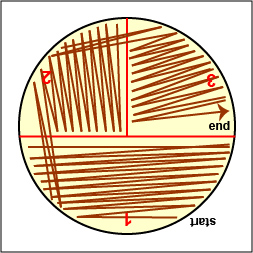
2. On the bottom of the petri plate, divide the plate into thirds with your wax marker and label as shown below. This will guide your streaking.
3. Lift the edge of the lid just enough to insert the loop.
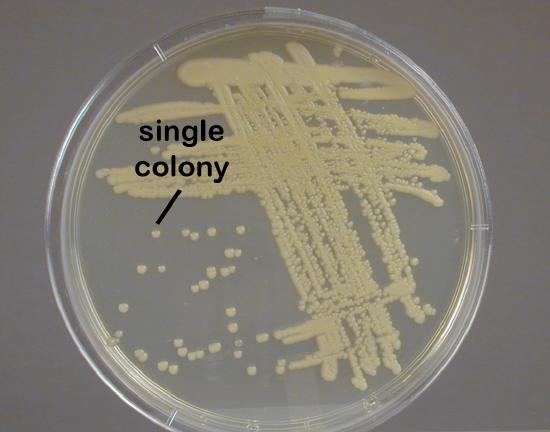
4. Streak the loop across the surface of the agar medium using the either the pattern shown in Fig. 2.5.2 or the pattern shown in Fig. 2.5.3. These streaking patterns allow you to obtain single isolated bacterial colonies originating from a single bacterium or arrangement of bacteria (see Fig. 2.5.4 ).
|
Fig. 2.5.2B: Streaking For Isolation, Step-2 |
Fig. 2.5.2C: Streaking For Isolation, Step-3 |
|
|---|---|---|
 |
 |
 |
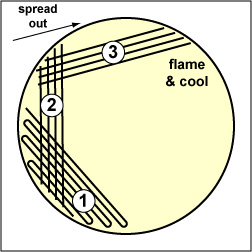
| Streak the inoculum over area "1" as shown above. Then flame the loop and cool it by sticking it into the edge of the agar. Rotate the plate 90 degrees counterclockwise. | Rotate the plate so area "1" is on your left. Drag your stetile inoculating loop through area "1" two times and spread out over area "2" as shown above. Then flame the loop and cool it by sticking it into the edge of the agar. Rotate the plate 90 degrees counterclockwise. | Rotate the plate so that area "2" is on your left. Drag your sterile inoculating loop through area "2" two times and spread the inoculum on area "2" over area "3." Sterilize the loop. |
| (Copyright; Gary E. Kaiser, Ph.D. The Community College of Baltimore County, Catonsville Campus CC-BY-3.0) | ||
|
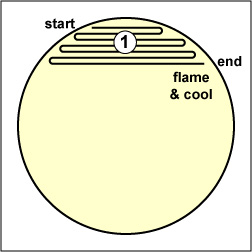
Fig. 2.5.2D: Streaking For Isolation, Step-1 |
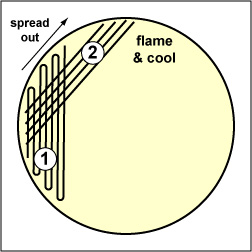
Fig. 2.5.2E: Streaking For Isolation, Step-2 |
Fig. 2.5.2F: Streaking For Isolation, Step-3 |
|---|---|---|
 |
 |
|
|
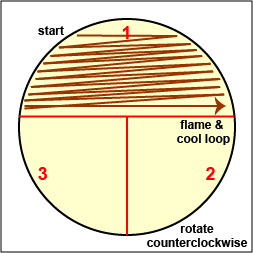
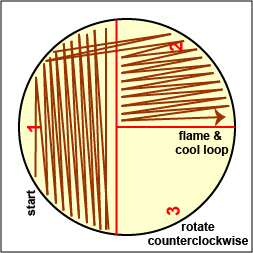
Streak the inoculum over area "1." Flame the loop and cool it by sticking it into the edge of the agar |
Spread the inoculum on area "1" over area "2" using 4-5 separate straight lines. Flame the loop and cool it by sticking it into the edge of the agar. |
Spread the inoculum on area "2" over area "3" using 4-5 separate straight lines. Flame the loop and cool it by sticking it into the edge of the agar. |
| (Copyright; Gary E. Kaiser, Ph.D. The Community College of Baltimore County, Catonsville Campus CC-BY-3.0) | ||
|
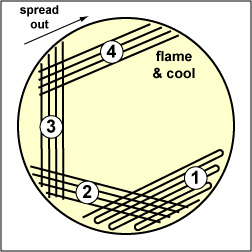
Fig. 2.5.3B : Streaking For Isolation, Step-5 |
Fig. 2.5.4 : Streaking For Isolation, Step-3 |
|
|---|---|---|
 |
 |
 |
|
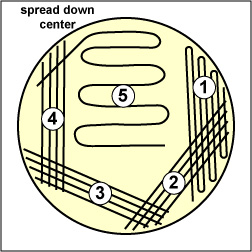
Spread the inoculum on area "3" over area "4" using 4-5 separate straight lines. |
Draw your loop through area "4" and spread it down the center of the plate without touching any of the areas already streaked. | Note Isolated colonies. |
| (Copyright; Gary E. Kaiser, Ph.D. The Community College of Baltimore County, Catonsville Campus CC-BY-3.0) | ||
In order to avoid digging into the agar as you streak the loop over the top of the agar you must keep the loop parallel to the the agar surface. Always start streaking at the "12:00 position" (See Fig. 2.5.2A .) of the plate and streak side-to-side as you pull the loop toward you. As you follow either Fig. 2.5.2 or Fig. 2.5.3, each time you flame and cool the loop between sectors, rotate the plate counterclockwise so you are always working in the "12:00 position" of the plate. This keeps the inoculating loop parallel with the agar surface and helps prevent the loop from digging into the agar.
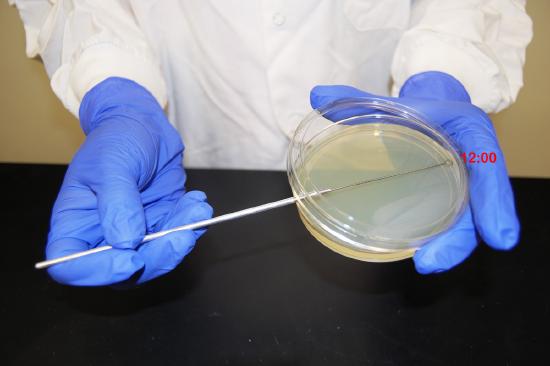
5. Remove the loop and immediately close the lid.
6. Resterilize the inoculating loop by placing it in the microincinerator for 10 seconds (see Fig. 2.5.5B ).

Video of an isolated streak plate
Look at the isolated colonies on three sector streak plates.
|
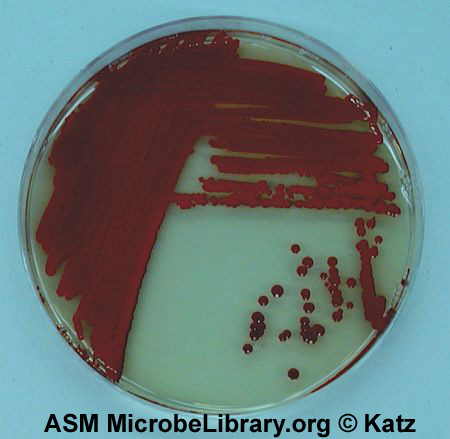
Fig. 2.5.6: Serratia marcescens on Trypticase Soy Agar; Three Sector Method |
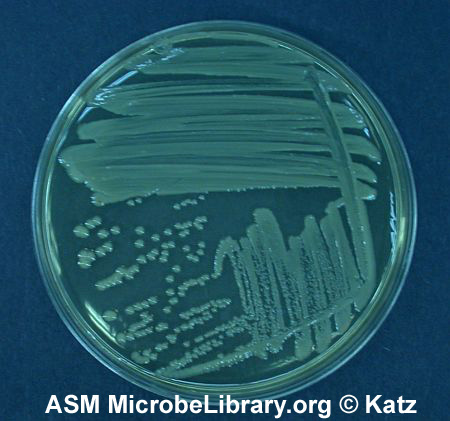
Fig. 2.5.7: Escherichia coli on Trypticase Soy Agar; Three Sector Method |
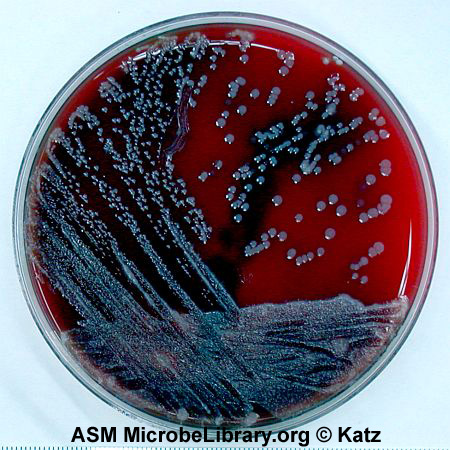
Fig. 2.5.8: Fecal Specimen on Blood Agar; Three Sector Method |
|---|---|---|
 |
 |
 |
|
Note Isolated colonies. Serratia marcescens on trypticase soy agar. © D. Sue Katz, author. Licensed for use, ASM MicrobeLibrary. |
Note Isolated colonies. Escherichia coli on trypticase soy agar. © D. Sue Katz, author. Licensed for use, ASM MicrobeLibrary. |
Note Isolated colonies. Fecal specimen on trypticase soy agar. © D. Sue Katz, author. Licensed for use, ASM MicrobeLibrary. |
In the future, every procedure in the lab will be done using similar aseptic technique
Contributors and Attributions
Dr. Gary Kaiser (COMMUNITY COLLEGE OF BALTIMORE COUNTY, CATONSVILLE CAMPUS)