Lab 5: Pipette and Environmental Requirements
- Page ID
- 23988
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Getting to Know Your Pipette:
1. Find the following parts on your pipette: 
- Volume adjustment dial
- Tip ejector button
- Plunger button
- Stainless steel micrometer
- Digital volume indicator
- Stainless steel ejector arm (removable)
- Plastic shaft
- Disposable yellow or blue tip
2. Practice holding your pipette correctly, placing a tip on our pipette, and ejected the tip. Do this at least three times.
3. The total volume a pipette can hold is stated on the top of the plunger. We will be working with the following three volume pipettes:
- P-20 0.02 μl – 20 μl
- P-200 20 μl – 200 μl
- P-1000 200 μl – 1000 μl
4. Based on the volume pipette you have the numbers in the digital display have a different meaning.

5. Rotate the volume adjustment knob until the digital indicator reaches the desired volume, then place a disposable tip on the shaft of the pipette (practice all three volumes min, int, max)
6. Press down on the plunger to the First Stop. (You will be able to push past this point, but there is enough resistance to stop the movement if you try to be aware of it.)
7. Hold the pipette vertically and immerse the disposable tip into the sample. Use the colored water and the microcentrifuge tubes provided to you.
8. Allow the plunger button to return slowly to its original position. Do not allow the button to snap up.
9. To dispense the sample: place the tip against the side wall of the receiving tube and push the plunger down to the first stop. Wait 2-3 seconds, then depress the plunger to the second stop in order to expel any residual sample in the tip.
10. While the plunger is still pushed down, remove the pipette from the tube and allow the plunger to slowly return to its original position.
11. Practice until you are ready and then call an instructor for your skills test. This skills test is worth 5 points.
Warning
- Never rotate the volume adjustment knob past the upper or lower range of the pipetman.
- Never lay the pipetman down on its side or hold it horizontally when it contains liquid.
- Never immerse the shaft of the pipetman into the fluid.
Environmental Requirements (Temperature)
How does temperature affect bacterial growth?
Organisms grow best over a certain temperature range, and this range has restrictions. The cardinal temperatures are the range of temperatures over which an organism can grow. Every organism has evolved to live at a particular optimum temperature.
- Minimum: lowest temp where reproduction occurs
- Maximum: highest temp where reproduction occurs
- Optimum: highest rate of reproduction
Organisms are classified based on the temperature ranges they live in:
- Psychrophiles: less than zero
- Psychrotrophs: 0-30°C
- Mesophiles: middle temperatures 15-45°C
- Thermophiles: 40-80°C
- Extreme Thermophiles: above 65°C

WHAT ARE THE CARDINAL TEMPERATURES OF 3 DIFFERENT ORGANISMS?
1. Working as a table you will need 15 Tryptic Soy Broth (TSB) tubes.
2. Label your broth tubes with the bacterial species (3 species) and temperature (5 temps) = 15 tubes:
- Escherichia coli (5 broth tubes)
- Geobacillus stearothermophilus (5 broth tubes)
- Pseudomonas fluorescens (5 broth tubes)
3. Mix each broth culture before using by gently tapping on the tube.
4. Using aseptic technique, use a sterile pipette to transfer 20 μl of each organism to appropriate test tube of broth.
Note
If you are not consistent with the volume you inoculate, your results will be undesirable.
5. Place all in the provided racks at the end of your table. When your table is done, one of you will need to place your rack in the 37°C incubator.
6. Each person at your table will streak for isolated colonies from a broth of Serratia marcescens onto a TSA plate.
7. Three people will incubate their plate at 30°C and the other half at 40°C. Containers for the plates will be placed on the instructor's desk.
Environmental Requirements (pH)
How does pH affect bacterial growth?
Hydrogen ions in a solution = pH. Organisms grow best at a specific pH range based, in part, on the environment they have evolved to live in. If bacteria are outside their optimal pH range their proteins can become denatured. Ranges of pH over which an organism can live place them in groups:
- Acidophiles: below pH 5.5
- Neutrophiles: pH 5.5 -8.5
- Alkaliphiles: pH above 8.5

WHAT ARE THE pH RANGES OF 3 DIFFERENT ORGANISMS?
8. Working as a table you will need 15 TSB tubes of the following pHs:
- 3 tubes of pH 2
- 3 tubes of pH 4
- 3 tubes of pH 6
- 3 tubes of pH 8
- 3 tubes of pH 10
9. Label your broth tubes with the bacterial species (3 species) and pH (5 pH) = 15 tubes
- Lactobacillus plantarum → pH 2, 4, 6, 8, 10
- Staphylococcus saprophyticus → pH 2, 4, 6, 8, 10
- Alcaligenes faecalis → pH 2, 4, 6, 8, 10
10. Mix the culture before using by gently tapping on the tube.
11. Using aseptic technique, use a sterile pipette to transfer 20 μl of each organism to appropriate test tube of broth.
12. Place all inoculated tubes in the provided racks at the end of your table. When your table is done, one of you will need to place that racks in the 37°C incubator.
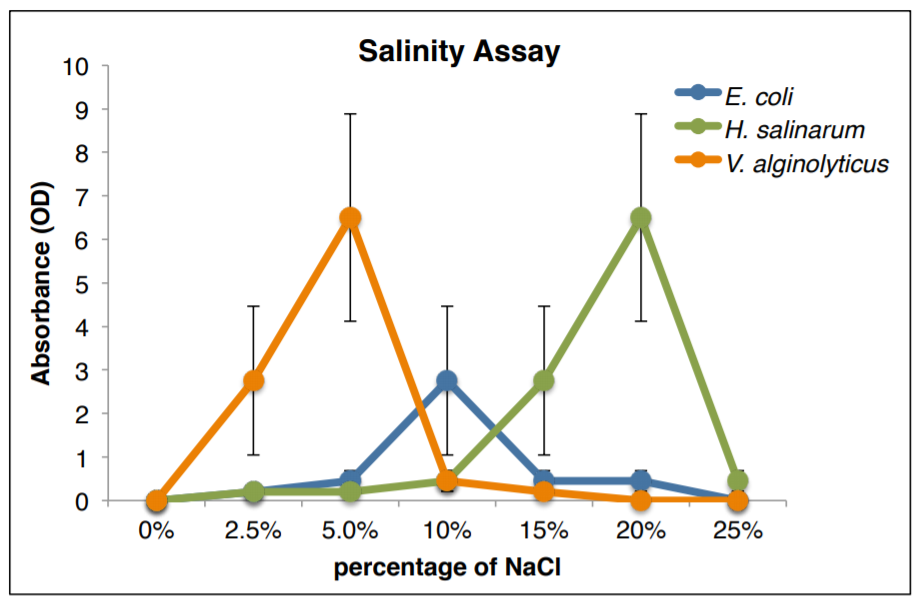
Environmental Requirements (Salinity)
How does osmotic pressure affect bacterial growth?
Water is essential to all organisms. The ability to control the movement of water across a membrane is necessary for the survival of all cells. Osmotic pressure is the minimum pressure which needs to be applied to a solution to prevent the inward flow of water across a semi-permeable membrane. The movement of water is controlled by the concentration of solutes contained within the water (usually salt). Bacteria can be classified based upon the salinity they can tolerate:
- Halophiles (prefer NaCl concentrations of 3% or higher)
- Extreme halophiles (prefer NaCl concentrations of 15%-25%)
- Xerophile (prefer low salt concentrations)

WHAT ARE THE PREFERRED SALINITY RANGES OF 4 DIFFERENT ORGANISMS?
11. Working as a table you will need 28 TSB broths of the following salinities:
- 4 tubes of NaCl 0%
- 4 tubes of NaCl 2.5%
- 4 tubes of NaCl 5%
- 4 tubes of NaCl 10%
- 4 tubes of NaCl 15%
- 4 tubes of NaCl 20%
- 4 tubes of NaCl 25%
12. Label your broth tubes with the bacterial species (4 species) and salinity (7 concentrations) = 28 tubes:
- Escherichia coli
- Halobacterium salinarum
- Staphylococcus epidermidis
- Vibrio alginolyticus
13. Mix the culture before using by gently tapping on the tube.
14. Using aseptic technique, use a sterile pipette to transfer 20 μl of each organism to appropriate test tube of broth.
15. Incubate E. coli and S. epidermidis at 37°C in the racks at your table, H. salinarum at 42°C, and V. alginolyticus at 30°C. These racks will be on the instructor's desk.
Environmental Requirements (Aerotolerance)
What is aerotolerance and how does it affect bacterial growth?
Bacteria can differ dramatically in their ability to utilize oxygen (O2). Under aerobic conditions, oxygen acts as the final electron acceptor for the electron transport chain located in the plasma membrane of prokaryotes. Bacteria use this process to generate ATP, the energy source for most cellular processes. In the absence of oxygen (O2), some bacteria can use alternative metabolic pathways including anaerobic respiration and/or fermentation. During anaerobic respiration, other alternative molecules are used as the final electron acceptor for the electron transport chain such as nitrate (NO3), sulfate (SO4), and carbonate (CO3).

WHAT IS THE AEROTOLERANCE OF THREE DIFFERENT SPECIES OF BACTERIA?
16. Working as a table you will need 2 yeast+glucose plates.
17. You will be using the following three bacterial species:
Alcaligenes faecalis
Staphylococcus epidermidis
Clostridium sporogenes 
18. Divide both plates into three sections and aseptically transfer each bacteria species to a section, use your loop to draw a circle instead of streaking.
You will be comparing the growth between the three sections so make sure your streaks are of equal size.
19. One plate will be placed in a Torbal Jar at 37°C (anaerobic conditions) and one plate will just go directly in the incubator at 37°C (aerobic conditions). The Torbal jar will be at the end of each table.
Environmental Requirements (Results):
How do we quantify bacterial growth?
Often in microbiology, we need to determine the number of bacterial cells in a broth. We can do this directly through spread plates (we will do this in a later lab) or indirectly by assessing the turbidity (cloudiness) of broth tubes. We measure turbidity using a spectrophotometer which gives us a reading of the light absorbance:
- more bacteria = more cloudy = higher absorbance
- less bacteria = less cloudy = lower absorbance

1. Make sure the screen of your spectrophotometer reads 600 nM followed by some set of numbers and then the letter A.
2. For the spectrophotometer reading, use a Kimwipe to wipe the outside of the “blank” tube. Each set of broths (temp, pH, salinity) will have their own blank tube
3. Place blank tube in the spectrophotometer, close the lid, and press the “0 ABS / 100% T” button. This will set the background level of absorbance present in the broth when there are no bacteria growing.
4. Remove the blank tube.
5. Mix each tube before using by gently tapping on the tube and wipe with Kimwipe.
6. Insert this tube into the spectrophotometer, and without pressing anything, record the absorbance.
7. Once you are finished add your data to the chart at the front of the class, you will need to copy the data for the entire class.
8. Calculate averages and standard deviation for each condition & make graphs for each bacteria per condition (refer to the example provided on page 36).
Environmental Requirements (Graphs)
You will now graph the average and standard deviation of your salinity data on a computer on whatever graphing program you are familiar with. Please make sure your graph resembles the one below and shows the following information:
- graph title
- axis labels with units
- vertical error bars ONLY
- average at each salinity
- all FOUR species of bacteria on one graph