14.2: Activating Antigen-Specific Cytotoxic T- Lymphocytes
- Page ID
- 3328
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- In terms of the role of cytotoxic T-lymphocytes (CTLs) in body defense:
- State from what cells cytotoxic T-lymphocytes are derived.
- Describe how they can react with and destroy virus-infected cells, cells containing intracellular bacteria, and cancer cells without harming normal cells. (Indicate the role of following: TCR, CD4, MHC-I, and peptides from endogenous antigens.)
- State the mechanism by which cytotoxic T-lymphocytes kill the cells to which they bind. (Indicate the role of the following: perforins, granzymes, caspases, and macrophages in the process.)
- Briefly describe two ways certain viruses may evade cell-mediated immunity.
Marking an Infected Cell or a Tumor Cell for Destruction by Cytotoxic T-Lymphocytes
One of the body's major defenses against viruses, intracellular bacteria, and cancers is the destruction of infected cells and tumor cells by cytotoxic T-lymphocytes (CTLs). These CTLs are effector cells derived from naive T8-lymphocytes during cell-mediated immunity. Both T8-lymphocytes and CTLs produce T-cell receptors or TCRs and CD8 molecules that are anchored to their surface.
- The TCRs and CD8 molecules on the surface of naive T8-lymphocytes are designed to recognize peptide epitopes bound to MHC-I molecules on antigen-presenting cells or APCs .
- The TCRs and CD8 molecules on the surface of cytotoxic T-lymphocytes (CTLs) are designed to recognize peptide epitopes bound to MHC-I molecules on infected cells and tumor cells.
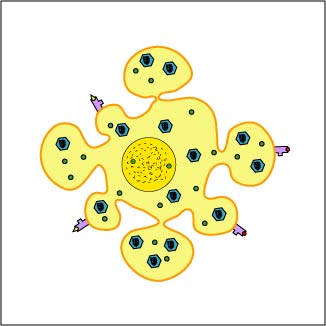
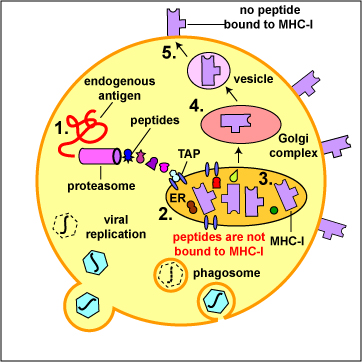
During the replication of viruses and intracellular bacteria within their host cell, as well as during the replication of tumor cells, viral, bacterial, or tumor proteins in the cytosol of that cell are degraded into a variety of peptide epitopes by cylindrical organelles called proteasomes . Other endogenous antigens such as proteins released into the cytosol from the phagosomes of antigen-presenting cells, such as macrophages and dendritic cells as well, as a variety of the human cell's own proteins (self-proteins) are also degraded by proteasomes. As these various endogenous antigens pass through proteasomes, proteases and peptidases chop the protein up into a series of peptides, typically 8-11 amino acids long (Figure \(\PageIndex{1}\)).
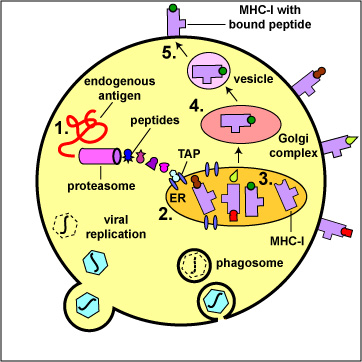

During the replication of viruses and intracellular bacteria within their host cell, as well as during the replication of tumor cells, viral, bacterial, or tumor proteins, as well proteins released from phagosomes of phagocytes and various human cell or self-proteins, are degraded into a variety of peptide epitopes by cylindrical organelles called proteasomes. As endogenous antigens pass through proteasomes, proteases and peptidases chop the protein up into a series of peptides, typically 8-11 amino acids long.
A transporter protein called TAP located in the membrane of the cell's endoplasmic reticulum then transports these peptide epitopes into the endoplasmic reticulum where they bind to the grooves of various newly made MHC-I molecules. The MHC-I molecules with bound peptides are then transported to the Golgi complex and placed in exocytic vesicles. The exocytic vesicles carry the MHC-I/peptide complexes to the cytoplasmic membrane of the cell where they become anchored to its surface (Figure \(\PageIndex{2}\)). A single cell may have up to 250,000 molecules of MHC-I with bound epitope on its surface.
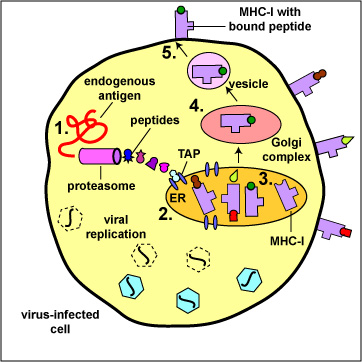
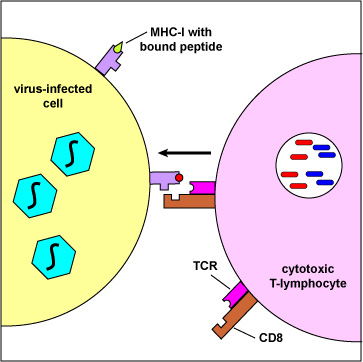
During cell-mediated immunity, MHC-I molecule with bound peptide on the surface of infected cells and tumor cells can be recognized by a complementary-shaped TCR/CD8 on the surface of a cytotoxic T-lymphocyte (CTL) to initiate destruction of the cell containing the endogenous antigen (Figure \(\PageIndex{3}\)).
Endogenous antigens are those located within the cytosol of cells of the body. Examples include:
- viral proteins produced during viral replication,
- proteins produced by intracellular bacteria such as Rickettsias and Chlamydias during their replication,
- proteins that have escaped into the cytosol from the phagosome of phagocytes such as antigen-presenting cells
- tumor antigens produced by cancer cells,
- and self peptides from human cell proteins.
The body marks infected cells and tumor cells for destruction by placing peptide epitopes from these endogenous antigens on their surface by way of MHC-I molecules. Cytotoxic T-lymphocytes (CTLs) are then able to recognize peptide/MHC-I complexes by means of their T-cell receptors (TCRs) and CD8 molecules and kill the cells to which they bind.
- Endogenous antigens, such as viral proteins, pass through proteasomes where they are degraded into a series of peptides.
- The peptides are transported into the rough endoplasmic reticulum (ER) by a transporter protein called TAP.
- The peptides then bind to the grooves of newly synthesized MHC-I molecules.
- The endoplasmic reticulum transports the MHC-I molecules with bound peptides to the Golgi complex.
- The Golgi complex, in turn, transports the MHC-I/peptide complexes by way of an exocytic vesicle to the cytoplasmic membrane where they become anchored. Here, the peptide and MHC-I/peptide complexes can be recognized by CTLs by way of TCRs and CD8 molecules having a complementary shape.
Cytotoxic T-Lymphocyte (CTL) Destruction of Body Cells Displaying Epitopes of Foreign Antigen on their Surface
The cytotoxic T-lymphocytes (CTLs) produced during cell-mediated immunity are designed to remove body cells displaying "foreign" epitope, such as virus-infected cells, cells containing intracellular bacteria, and cancer cells with mutant surface proteins. The CTLs are able to kill these cells by inducing a programmed cell death known as apoptosis. Using virus-infected cells as an example, the CTLs circulate throughout the body where they encounter virus-infected cells and induce apoptosis. This involves involves a complex of intracellular cytotoxic granules containing:
- Pore-forming proteins called perforins
- Proteolytic enzymes called granzymes and
- Granulysin
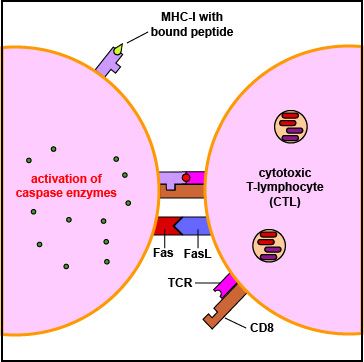
When the TCR and CD8 of the CTL binds to the MHC-I/epitope on the surface of the virus-infected cell or tumor cell (Figure \(\PageIndex{4}\)), this sends a signal through a CD3 molecule which triggers the release of the cytotoxic perforins/granzymes/granulysin granules from the CTL.
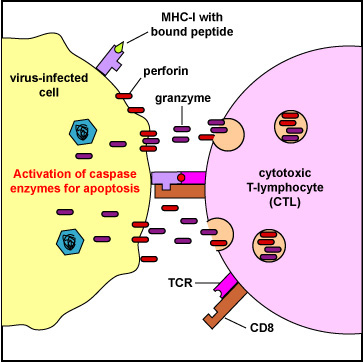
The exact mechanism of entry of the granzymes into the infected cell or tumor cell is still debated. It is, however, dependent on perforins. Possibilities include:
- The perforins/granzymes/granulysin complex may be taken into the target cell by receptor-mediated endocytosis. The perforin molecules may then act on the endosomal membrane allowing granzymes to enter the cytosol.
- The perforin molecules may put pores in the membrane of the target cell allowing the granzymes to directly enter the cytosol (Figure \(\PageIndex{5}\)).
Killing of the infected cell or tumor cell by apoptosis involves a variety of mechanisms:
- Certain granzymes can activate the caspase enzymes that lead to apoptosis of the infected cell. The caspases are proteases that destroy the protein structural scaffolding of the cell - the cytoskeleton - and nucleases that degrade both the target cell's nucleoprotein and any microbial DNA within the cell (Figure \(\PageIndex{5}\)).
- Granzymes cleave a variety of other cellular substrates that contribute to cell death.
- The perforin molecules may also polymerize and form pores in the membrane of the infected cell, similar to those produced by MAC. This can increase the permeability of the infected cell and contribute to cell death. If enough perforin pores form, the cell might not be able to exclude ions and water and may undergo cytolysis.
- Granulysin has antimicrobial actions and can also induce apoptosis.
- Electron micrograph of a CTL binding to a tumor cell.
- Electron micrograph showing a killed tumor cell.
- CTLs can also trigger apoptosis through FasL/Fas interactions. Activated lymphocytes express both death receptors called Fas and Fas ligand or FasL (Figure \(\PageIndex{6}\)) on their surface. This FasL/Fas interaction triggers an intracellular transduction that activates the caspase enzymes that lead to apoptosis. In this way, CTLs can kill other lymphocytes and terminate lymphocyte proliferation after the immune responses have eradicated an infection.
Death by apoptosis does not result in the release of cellular contents such as inflammatory mediators or viruses as occurs during immune-induced cell lysis. Instead, the cell breaks into membrane-bound apoptoptic fragments that are subsequently removed by macrophages. This reduces inflammation and also prevents the release of viruses that have assembled within the infected cell and their spread into uninfected cells. Since the CTLs are not destroyed in these reactions, they can function over and over again to destroy more virus-infected cells.
- Some viruses inhibit proteasomal activity in the cells they infect. Explain specifically how this might better enable the virus to resist adaptive immunity.
- Some viruses suppress the production of MHC-I molecules in the cells they infect. Explain specifically how this might better enable the virus to resist adaptive immunity.
- Some viruses block the TAP transport of peptides into the endoplasmic reticulum of the cells they infect. Explain specifically how this might better enable the virus to resist adaptive immunity.
- Some viruses block apoptosis of the cells they infect. Explain specifically how this might better enable the virus to resist adaptive immunity.
As with humoral immunity, certain microbes are able to evade to some degree cell-mediated immunity:
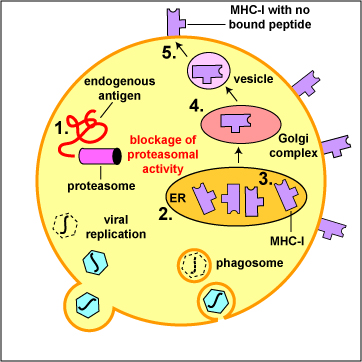
- Epstein-Barr virus (EBV) and cytomegalovirus (CMV) inhibit proteasomal activity so that viral proteins are not degraded into viral peptides. (see Figure \(\PageIndex{7}\)A)
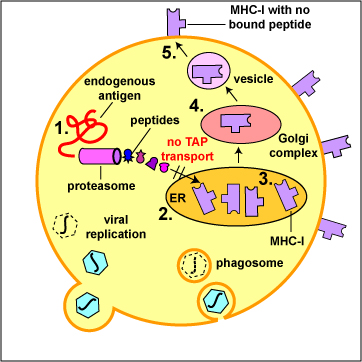
- Herpes simplex viruses (HSV) can block the TAP transport of peptides into the endoplasmic reticulum (see Figure \(\PageIndex{7}\)B).
- Numerous viruses, such as the cytomegalovirus (CMV) and adenoviruses can block the formation of MHC-I molecules by the infected cell. As a result, no viral peptide is displayed on the infected cell and the CTLs are no longer able to recognize that the cell is infected and kill it (see Figure \(\PageIndex{7}\)C).
- Epstein-Barr virus (EBV) down regulates several host proteins involved in attaching viral epitopes to MHC-I molecules and displaying them on the host cell's surface (see Figure \(\PageIndex{7}\)D).
- Adenoviruses and Epstein-Barr Viruses (EBV) code for proteins that blocks apoptosis, the programmed cell suicide mechanism triggered by various defense mechanisms in order to destroy virus-infected cells.
Summary
- Cell-mediated immunity (CMI) is an immune response that does not involve antibodies but rather involves the activation of macrophages and NK-cells, the production of antigen-specific cytotoxic T-lymphocytes, and the release of various cytokines in response to an antigen.
- Cell-mediated immunity is directed primarily microbes that survive in phagocytes and microbes that infect non-phagocytic cells.
- One of the body's major defenses against viruses, intracellular bacteria, and cancers is the destruction of infected cells and tumor cells by cytotoxic T-lymphocytes or CTLs, effector cells derived from naïve T8-lymphocytes during cell-mediated immunity.
- The TCRs and CD8 molecules on the surface of naive T8-lymphocytes are designed to recognize peptide epitopes bound to MHC-I molecules on antigen-presenting cells (APCs).
- During the replication of viruses and intracellular bacteria within their host cell, as well as during the replication of tumor cells, viral, bacterial, or tumor proteins (endogenous antigens) in the cytosol of that cell are degraded into a variety of peptide epitopes by cylindrical organelles called proteasomes.
- These peptide epitopes bind to MHC-I molecules being synthesized in the endoplasmic reticulum which are eventually transported to the cytoplasmic membrane of that cell.
- During cell-mediated immunity, MHC-I molecule with bound peptide on the surface of infected cells and tumor cells can be recognized by a complementary-shaped TCR/CD8 on the surface of a cytotoxic T-lymphocyte (CTL) to initiate destruction of the cell containing the endogenous antigens.
- When the TCR and CD8 of the CTL binds to the MHC-I/epitope on the surface of the virus-infected cell or tumor cell, this triggers the release of cytotoxic perforins/granzymes/ granulysin granules from the CTL that lead to apoptosis, a programmed cell suicide of that cell.
- Cell death by apoptosis does not result in the release of cellular contents such as inflammatory mediators or viruses as occurs during immune-induced cell lysis.
- During apoptosis, the cell breaks into membrane-bound apoptotic fragments that are subsequently removed by macrophages.


