17.2A: Carbon Cycle
- Page ID
- 5820
The concentration of carbon in living matter (18%) is almost 100 times greater than its concentration in the earth (0.19%). So living things extract carbon from their nonliving environment. For life to continue, this carbon must be recycled. Carbon exists in the nonliving environment as:
- carbon dioxide (CO2) in the atmosphere and dissolved in water (forming HCO3−)
- carbonate rocks (limestone and coral = CaCO3)
- deposits of coal, petroleum, and natural gas derived from once-living things
- dead organic matter, e.g., humus in the soil
Carbon enters the biotic world through the action of primarily photoautotrophs, like plants and algae, that use the energy of light to convert carbon dioxide to organic matter and to a small extent, chemoautotrophs - bacteria and archaea that do the same but use the energy derived from an oxidation of molecules in their substrate. Carbon returns to the atmosphere and water by respiration (as CO2), burning, and decay (producing CO2 if oxygen is present, methane (CH4) if it is not.
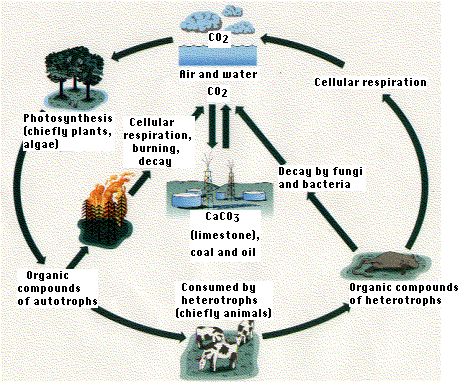
The uptake and return of CO2 are not in balance
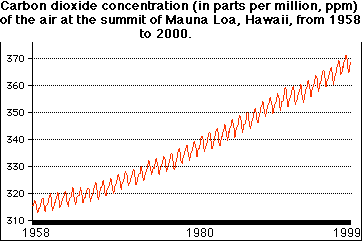
The carbon dioxide content of the atmosphere is gradually and steadily increasing. The graph shows the CO2 concentration at the summit of Mauna Loa in Hawaii from 1958 through 1999. The values are in parts per million (ppm). The seasonal fluctuation is caused by the increased uptake of CO2 by plants in the summer. (In March 2015, its average worldwide concentration reached 400 ppm.)
The increase in CO2 probably began with the start of the industrial revolution. Samples of air trapped over the centuries in the glacial ice of Greenland show no change in CO2 content until 300 years ago. Since measurements of atmospheric CO2 began late in the nineteenth century, its concentration has risen over 20%. This increase is surely "anthropogenic"; that is, caused by human activities:
- burning fossil fuels (coal, oil, natural gas) which returns to the atmosphere carbon that has been locked within the earth for millions of years.
- clearing and burning of forests, especially in the tropics. In recent decades, large areas of the Amazon rain forest have been cleared for agriculture and cattle grazing.
Where is the missing carbon?
Curiously, the increase in atmospheric CO2 is only about one-half of what would have been expected from the amount of fossil fuel consumption and forest burning. Where has the rest gone? Research has shown that increased CO2 levels lead to increased net production by photoautotrophs. There is evidence that at least some of the missing CO2 has been incorporated by: (1) increased growth of forests, especially in North America, (2) increased amounts of photoautotrophic plankton in the oceans, and (3) uptake by desert soils (mechanism as yet unknown)
The Greenhouse Effect and Global Warming
Despite these "sinks" for our greatly-increased CO2 production, the concentration of atmospheric CO2 continues to rise? Should we be worried? Carbon dioxide is transparent to light but rather opaque to heat rays. Therefore, CO2 in the atmosphere retards the radiation of heat from the earth back into space - the "greenhouse effect". Has the increase in carbon dioxide led to global warming?
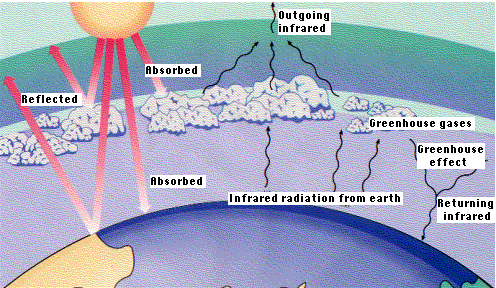
Some evidence:
- Careful monitoring shows that the global air temperature in 2014 was 0.57°C higher than the average from 1961–1990, and that 14 of the 15 warmest years since records began being kept late in the 19th Century have occurred in this century (including 2005 and 2010 as well as 2014).
- Many glaciers and ice sheets are receding.
- Woody shrubs are now growing in areas of northern Alaska that 50 years ago were barren tundra.
- Many angiosperms in temperate climates are flowering earlier in the spring than they used to.
- Many species of birds and butterflies are moving north and breeding earlier in the spring.
Will continued increase in carbon dioxide lead to more global warming and, if so, how much? At this point, the answer depends on what assumptions you plug into your computer models. But as the different models have been improved, they seem to be converging on a consensus: a doubling of the CO2 concentration (expected by the end of this century) will cause the earth to warm somewhere in the range of 1.1–6.4°C.
Other Greenhouse Gases
Although their levels in the atmosphere are much lower than that of CO2,
- methane (CH4)
- nitrous oxide (N2O)
- hydrofluorocarbons (HFCs)
are also potent greenhouse gases.
Methane
Although methane ("marsh gas") is released by natural processes (e.g. from decay occurring in swamps), human activities now account for some 60% of the total.
- mining, processing, and use of coal, oil, and natural gas
- release from landfills
- growing rice in paddies
- burning forests
- raising cattle (fermentation in their rumens produces methane that is expelled from their GI tract)
So burning of the tropical rain forest adds to the atmospheric methane budget in two ways:
- incomplete combustion during burning
- release from the GI tract of the cattle that are later placed on the cleared land.
The methane concentration in Arctic air is presently some 1.9 parts per million, the highest level seen such measurements began. Although this concentration is far less than that of CO2, methane is 28 times as potent a greenhouse gas. The marked warming of the earth that occurred at the end of the Paleocene epoch is thought to have been caused by the release of large amounts of methane from the sea floor.


