16.5B: Auxin
- Page ID
- 5799
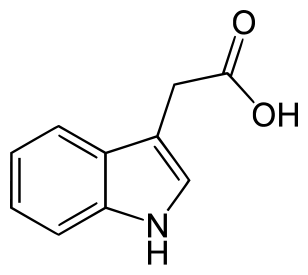
Auxins are plant hormones. The most important auxin produced by plants is indole-3-acetic acid (IAA). It plays important roles in a number of plant activities, including:
- development of the embryo
- leaf formation
- phototropism
- gravitropism
- apical dominance
- fruit development
- abscission
- root initiation and development
- the shade-avoidance effect
Embryonic Development
From the very first mitotic division of the zygote, gradients of auxin guide the patterning of the embryo into the parts that will become the organs of the plant:
- shoot apex,
- primary leaves,
- cotyledon(s),
- stem,
- root.
Leaf Formation
The formation of new leaves in the apical meristem is initiated by the accumulation of auxin. Already-developing leaves deplete the surrounding cells of auxin so that the new leaves do not form too close to them. In this way, the characteristic pattern of leaves in the plant is established. Auxin also controls the precise patterning of the epidermal cells of the developing leaf.
Phototropism
Plant shoots display positive phototropism: when illuminated from one direction, the shoot proceeds to grow in that direction. Proposed Mechanism for phototropism is a multiple process. The direction of light is detected at the tip of the shoot with (blue light is most effective). It is absorbed by a flavoprotein called phototropin. Flavoproteins contain flavin as a prosthetic group. Auxin moves from the tip down. An auxin transporter - one of the PIN proteins - is inserted in the plasma membrane at the lateral face of cells of the shoot. Auxin is pumped out of these efflux transporters and accumulates in the cells on the shady side. This stimulates elongation of the cells on the shady side causing the shoot to bend toward the light.
Gravitropism
Gravitropism is a plant growth response to gravity.
- Plant shoots display negative gravitropism: when placed on its side, a plant shoot will grow up
- Roots display positive gravitropism: they grow down.
Possible Mechanism of Gravitropism in Roots
When a root is placed on its side,
- Statoliths (organelles containing starch grains) settle by gravity to the bottom of cells in the root tip.
- This causes PIN proteins to redistribute to the underside of the cell where they pump auxin out of the cell; that is, they are efflux transporters.
- The auxin then accumulates along the under side of the root.
- This INHIBITS root cell elongation.
- So the cells at the top surface of the root elongate, causing the root to grow down.
Apical Dominance
Growth of the shoot apex (terminal shoot) usually inhibits the development of the lateral buds on the stem beneath. This phenomenon is called apical dominance. If the terminal shoot of a plant is removed, the inhibition is lifted, and lateral buds begin growth. Gardeners exploit this principle by pruning the terminal shoot of ornamental shrubs, etc. The release of apical dominance enables lateral branches to develop and the plant becomes bushier. The process usually must be repeated because one or two laterals will eventually outstrip the others and reimpose apical dominance.
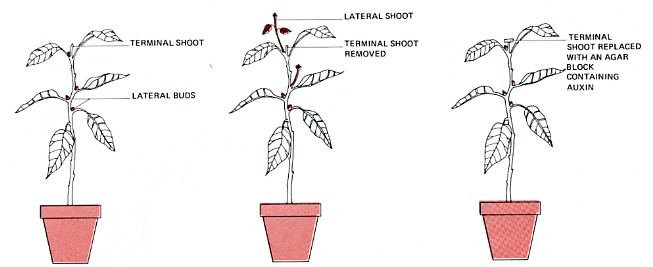
Apical dominance seems to result from the downward transport of auxin produced in the apical meristem. In fact, if the apical meristem is removed and IAA applied to the stump, inhibition of the lateral buds is maintained.
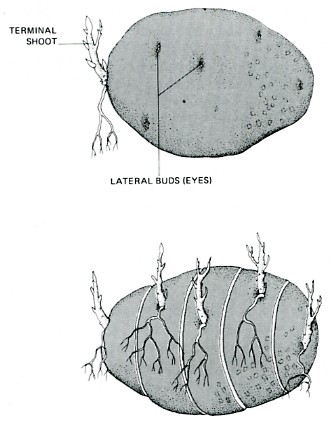
The common white potato is really a portion of the underground stem of the potato plant. It has a terminal bud or "eye" and several lateral buds. After a long period of storage, the terminal bud usually sprouts but the other buds do not. However, if the potato is sliced into sections, one bud to a section, the lateral buds develop just as quickly as the terminal bud.
Fruit Development
Pollination of the flowers of angiosperms initiates the formation of seeds. As the seeds mature, they release auxin to the surrounding flower parts, which develop into the fruit that covers the seeds.
Some commercial growers deliberately initiate fruit development by applying auxin to the flowers. Not only does this ensure that all the flowers will "set" fruit, but it also maximizes the likelihood that all the fruits will be ready for harvest at the same time.
Abscission
Auxin also plays a role in the abscission of leaves and fruits. Young leaves and fruits produce auxin and so long as they do so, they remain attached to the stem. When the level of auxin declines, a special layer of cells — the abscission layer — forms at the base of the petiole or fruit stalk. Soon the petiole or fruit stalk breaks free at this point and the leaf or fruit falls to the ground.
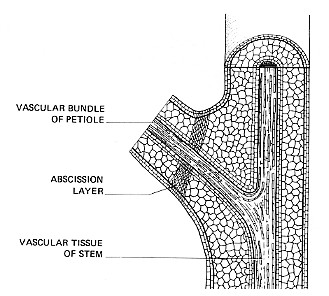
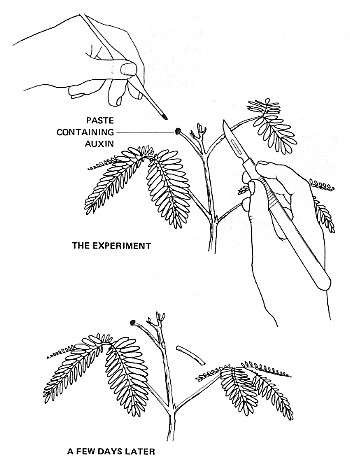
The figure on the right shows a nice demonstration of the role of auxin in abscission. If the blade of the leaf is removed, as shown in the figure, the petiole remains attached to the stem for a few more days. The removal of the blade seems to be the trigger as an undamaged leaf at the same node of the stem remains on the plant much longer, in fact, the normal length of time. If, however, auxin is applied to the cut end of the petiole, abscission of the petiole is greatly delayed.
Fruit growers often apply auxin sprays to cut down the loss of fruit from premature dropping.
Root Initiation and Development
The localized accumulation of auxin in epidermal cells of the root initiates the formation of lateral or secondary roots. Auxin also stimulates the formation of adventitious roots in many species. Adventitious roots grow from stems or leaves rather than from the regular root system of the plant.
Horticulturists may propagate desirable plants by cutting pieces of stem and placing them base down in moist soil. Eventually adventitious roots grow out at the base of the cutting. The process can often be hastened by treating the cuttings with a solution or powder containing a synthetic auxin.
Once a root is formed, a gradient of auxin concentration develops highest at the tip promoting the production of new cells at the meristem, and lowest in the region of differentiation, thus promoting the elongation and differentiation of root cells. The drop in auxin activity in the regions of elongation and differentiation is mediated by cytokinin — an auxin antagonist.
Translocation of Auxin
Auxin moves through the plant by two mechanisms: It passes in the sap moving through the phloem from where it is synthesized (its "source", usually the shoot) to a "sink" (e.g., the root). It also passes from cell to cell by the following mechanism.
Auxin can enter the cell by diffusion and also through influx transporters in the plasma membrane. It moves out through efflux transporters - called PIN proteins. Eight different types of PIN proteins have been identified so far. These are transmembrane proteins inserted in localized portions of the plasma membrane, e.g.,
- at the top of the cell where they move auxin toward the top of the plant;
- at the basal surface of the cell where they move auxin down the plant;
- at the lateral surface of the cell where they move auxin laterally (e.g., to mediate phototropism and gravitropism).
Identifying the signals that direct the appropriate placement of the PIN proteins is an active area of research.
How does auxin achieve its many different effects in the plant? Auxin effects are mediated by two different pathways: immediate, direct effects on the cell and turning on of new patterns of gene expression
Direct effects of auxin
The arrival of auxin in the cytosol initiates such immediate responses as
- changes in the concentration of and movement of ions in and out of the cell
- reduction in the redistribution of PIN proteins
Some of the direct effects of auxin may be mediated by its binding to a cell-surface receptor designated ABP1 ("Auxin-binding protein 1").
Effects of auxin on gene expression
Many auxin effects are mediated by changes in the transcription of genes. Auxin enters the nucleus and binds to its receptor, a protein called TIR1 ("transport inhibitor response protein 1") which now can bind to proteins responsible for attaching ubiquitin to one or another of several Aux/IAA proteins. This triggers the destruction of the Aux/IAA proteins by proteasomes. Aux/IAA proteins normally bind transcription factors called auxin response factors (ARF) preventing them from activating the promoters and other control sequences of genes that are turned on (or off) by auxin. Destruction of the Aux/IAA proteins relieves this inhibition, and gene transcription begins.
This mechanism is another of the many cases in biology where a pathway is turned on by inhibiting the inhibitor of that pathway (a double-negative is a positive). For example, the gibberellins, another group of plant hormones, exert their effects using a similar strategy. The presence in the cell of many different Aux/IAA proteins (29 in Arabidopsis), many different ARFs (23 in Arabidopsis) and several (~4) TIR1-like proteins provides a logical basis for mediating the different auxin effects that I have described. But how this is done remains to be discovered.
Synthetic auxins as weed killers
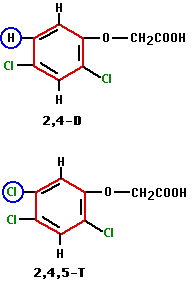
Some of the most widely-used weed killers are synthetic auxins. These include 2,4-dichlorophenoxy acetic acid (2,4-D) and 2,4,5-trichlorophenoxy acetic acid (2,4,5-T). As the formulas show, 2,4,5-T is 2,4-D with a third chlorine atom, instead of a hydrogen atom, at the #5 position in the benzene ring (blue circles).
2,4-D and its many variants are popular because they are selective herbicides, killing broad-leaved plants but not grasses (no one knows the basis of this selectivity).
Why should a synthetic auxin kill the plant? It turns out that the auxin influx transporter works fine for 2,4-D, but that 2,4-D cannot leave the cell through the efflux transporters. Perhaps it is the resulting accumulation of 2,4-D within the cell that kills it.
A mixture of 2,4,-D and 2,4,5-T was the "agent orange" used by the U.S. military to defoliate the forest in parts of South Vietnam. Because of health concerns, 2,4,5-T is no longer used in the U.S.


