15.8E: Drugs and the Nervous System
- Page ID
- 5650
The activity of the nervous system is mediated by many kinds of interneurons releasing one or another neurotransmitter such as
- noradrenaline
- gamma aminobutyric acid (GABA)
- dopamine
- glutamate (Glu)
- acetylcholine (ACh)
- serotonin
Presynaptic neurons synthesize and package their neurotransmitter in vesicles for release (by exocytosis) at the synapse. They often have "reuptake" transporters that reclaim the transmitter back into the cell when it has done its job. Postsynaptic neurons display receptors to which the neurotransmitter binds. All of this machinery provides many targets for alteration by exogenous chemicals; that is, psychoactive chemicals introduced into the body. These drugs fall into several distinct families.
Stimulants
The most widely used stimulants are
- caffeine (in coffee, tea, and cola beverages)
- nicotine (in cigarettes)
- amphetamines
- cocaine
All of these drugs mimic the stimulation provided by the sympathetic nervous system.
Nicotine binds to a subset of acetylcholine (ACh) receptors. ACh is a neurotransmitter at synapses early in the pathways of sympathetic stimulation. Although a weak drug in one sense, nicotine is strongly addictive. The use of e-cigarettes, chewing gum and skin patches containing nicotine is designed to satisfy the craving for nicotine while avoiding the serious health effects of other ingredients in cigarette smoke.
Amphetamines and cocaine bind to — thus blocking — transporters used for the reuptake of dopamine (and noradrenaline) into presynaptic neurons. This causes the level of dopamine to rise in the synapses. High levels of dopamine in an area of the brain called the nucleus accumbens appear to mediate the pleasurable effects associated with these (as well as other) psychoactive drugs.
| Generic name | Trade name |
|---|---|
| dextroamphetamine sulfate | Dexedrine |
| methylphenidate | Ritalin |
| pemoline | Cylert |
| mixture of 4 amphetamines | Adderall |
The chief medical uses for amphetamines and amphetamine-like drugs are to help people lose weight (because they suppress appetite) and to help children with attention deficit/hyperactivity disorder (ADHD) to perform better in school. At first glance, this second use seems counterproductive. This controversial procedure seems to work by increasing the alertness of the child so that it can focus its energies more effectively on the tasks in front of it.
Fen-Phen
Fen-Phen refers to a mixture of two amphetamine-like drugs fenfluramine and phentermine that were prescribed for losing weight. Because of reports of occasional very serious side effects, the mixture is no longer available and fenfluramine has been removed from the U.S. market.
Cocaine
Cocaine has been used for thousands of years by certain tribes in the Andes of South America. Cocaine and some of its relatives have legitimate medical uses as local anesthetics (e.g., lidocaine). However, the widespread recreational use of cocaine has created serious social problems. In order to achieve its effects, cocaine must cross the so-called blood-brain barrier. If antibodies are bound to the cocaine molecule, it cannot cross. This has raised the possibility of immunizing people against cocaine. It works in mice.
Sedatives
Sedatives induce sleep. They include
- ethanol (beverage alcohol)
- barbiturates, such as
- phenobarbital
- secobarbital (Seconal®)
- meprobamate (Miltown®, Equanil®)
Ethanol
Ethyl alcohol (ethanol) is, by a wide margin, the most widely used drug in most of the world. Its popularity comes not from its sedative effect but from the sense of well-being that it induces at low doses. Perhaps low doses sedate those parts of the brain involved with, for example, tension and anxiety and in this way produce a sense of euphoria. However, higher doses depress brain centers involved in such important functions as pain sensation, coordination, and balance. At sufficiently high doses, the reticular formation can be depressed enough to cause loss of consciousness.
Ethanol increases the release of the neurotransmitter GABA activating GABAA receptors and directly inhibits NMDA receptors.
Barbiturates
Barbiturates are often prescribed as sleeping pills and also to prevent seizures. Barbiturates mimic some of the action of ethanol, particularly in their ability to depress the reticular formation (thus promoting sleep) and, in high doses, the medulla oblongata (thus stopping breathing).
Barbiturates bind to a subset of GABA receptors designated GABAA receptors. These are ligand-gated channels that enhance the flow of chloride ions (Cl−) into the postsynaptic neuron, thus increasing its resting potential and making it less likely to fire. By binding to the GABAA receptor, barbiturates (and perhaps ethanol) increase the natural inhibitory effect of GABA synapses. Barbiturates and alcohol act additively — the combination producing a depression greater than either one alone. The combination is a frequent cause of suicide, both accidental and planned.
Meprobamate
Meprobamate is prescribed as a tranquilizer, but its action is quite different from the tranquilizers discussed below. Its molecular activity is like that of other sedatives and in combination with them can produce a lethal overdose. All sedatives produce two related physiological effects:
- tolerance — the necessity for a steadily-increasing dose to achieve the same physiological and psychological effects
- physical dependence — withdrawal of the drug precipitates unpleasant physical and psychological symptoms.
These traits are also shared with nicotine, opioids, and other psychoactive drugs.
Local Anesthetics
These chemical relatives of cocaine act by blocking the voltage-gated Na+ channels of sensory neurons preventing them from generating action potentials. They are injected or applied topically and block transmission not only in pain-conducting neurons but in others as well (causing general numbness).
Examples:
- lidocaine (Xylocaine®)
- procaine (Novocaine®)
Inhaled Anesthetics
Most of these are volatile hydrocarbons or ethers. Diethyl ether and chloroform are seldom used today, having been replaced by safer alternatives such as isofluorane, a fluorinated ether. Some, like isofluorane, bind to inhibitory GABA receptors) in the brain hyperpolarizing, and thus decreasing the sensitivity of, postsynaptic neurons. Others, like ketamine, block the activity of excitatory glutamate receptors.
Other Hydrocarbons
1,4-Butanediol is a common solvent. When ingested, it is converted into γ-hydroxybutyrate, an increasingly-popular (and illegal) "club drug". γ-Hydroxybutyrate acts on GABAB receptors. Conversion of 1,4-butanediol to γ-hydroxybutyrate requires the enzyme alcohol dehydrogenase, the same enzyme used to metabolize ethanol. Ingesting both ethanol and 1,4-butanediol delays the effects of the latter.
Opioids
These are substances isolated from the opium poppy or synthetic relatives. (They are also called opiates.)
Examples:
- morphine
- codeine
- heroin
- fentanyl (a synthetic that is ~80 times more potent than morphine)
- methadone
- oxycodone
Opioids depress nerve transmission in sensory pathways of the spinal cord and brain that signal pain. This explains why opioids are such effective pain killers. Opioids also inhibit brain centers controlling coughing, breathing, and intestinal motility. Both morphine and codeine are used as pain killers, and codeine is also used in cough medicine. Opioids are exceedingly addictive, quickly producing tolerance and dependence. Although heroin is even more effective as a painkiller than morphine and codeine, it is so highly addictive that its use is illegal. Methadone is a synthetic opioid that is used to break addiction to heroin (and replace it with addiction to methadone).
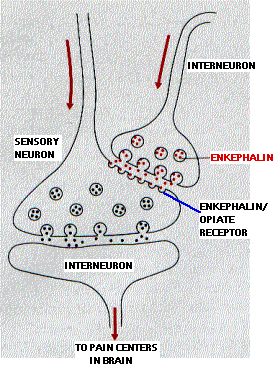
Opioids bind to so-called mu (µ) receptors . These G-protein-coupled receptors are located on the subsynaptic membrane of neurons involved in the transmission of pain signals. Their natural ligands are two pentapeptides (containing five amino acids):
- Met-enkephalin (Tyr-Gly-Gly-Phe-Met-COO-)
- Leu-enkephalin (Tyr-Gly-Gly-Phe-Leu-COO-)
Release of enkephalins suppresses the transmission of pain signals. Little is to be gained by having the perception of pain increase indefinitely in proportion to the amount of damage done to the body. Beyond a certain point, it makes sense to have a system that decreases its own sensitivity in the face of massive, intractable pain.
By binding to mu (µ) receptors, opioids like morphine enhance the pain-killing effects of enkephalin neurons. Opioid tolerance can be explained, at least in part, as a homeostatic response that reduces the sensitivity of the system to compensate for continued exposure to high levels of morphine or heroin. When the drug is stopped, the system is no longer as sensitive to the soothing effects of the enkephalin neurons and the pain of withdrawal is produced.
Mu (µ) receptors are also found on the cells in the medulla oblongata that regulate breathing. This accounts for the suppressive effect opioids have on breathing.
Opioid antagonists
Opioid antagonists such as naloxone (Narcan®) and naltrexone (ReVia®) bind to µ receptors but instead of activating them, they prevent the binding of the opioids themselves. In fact, if the receptors are already occupied by, for example, heroin molecules, naloxone will push the heroin molecules off and quickly rescue the patient from a drug overdose. Naltrexone is used to help recovering heroin addicts stay drug-free.
Antipsychotics
Antipsychotics (also called "neuroleptics") are used to treat schizophrenia, a common and devastating mental disease. They act by binding to one class of receptors for the neurotransmitter dopamine. There are two groups currently in use:
- "Typical" antipsychotics (sometimes referred to as "major tranquilizers"). Examples:
- chlorpromazine (Thorazine®)
- haloperidol (Haldol®)
- "Atypical" antipsychotics (also referred to as "second generation" antipsychotics). Examples:
- risperidone (Risperdal®)
- olanzapine (Zyprexa®)
- quetiapine (Seroquel®)
Tranquilizers
Tranquilizers act like sedatives in reducing anxiety and tensions. Most belong to a group called benzodiazepines and include such commonly-prescribed drugs as Xanax® and Klonopin®. The benzodiazepines act on interneurons that use the inhibitory neurotransmitter GABA. By binding to GABAA receptors on the postsynaptic membrane, they enhance the action of GABA at the synapse.This is the same receptor to which barbiturates (and perhaps ethanol) bind. Thus although benzodiazepines seem safe enough when used alone, combining them with ethanol or barbiturates can be (and often has been) lethal.
Antidepressants
Antidepressants fall into four chemical categories (of which we shall examine three). Most share a common property: they increase the amount of serotonin at synapses that use it as a neurotransmitter.
Monoamine oxidase inhibitors (MAOIs)
These drugs act on a mitochondrial enzyme that breaks down monoamines such as noradrenaline and serotonin. By inhibiting the enzyme in presynaptic serotonin-releasing neurons, more noradrenaline and serotonin is deposited in the synapse. Some examples: Parnate®, Nardil®, Marplan®. For several reasons, MAO inhibitors are not used much anymore.
Tricyclic antidepressants (TCAs)
These drugs block the reuptake of noradrenaline, dopamine, and serotonin causing an increase in the level of these neurotransmitters in the synapse.
Examples:
| Generic name | Trade name |
| imipramine | Tofranil® |
| clomiprimine | Anafranil® |
| amitriptyline | Elavil® |
Although tricyclics are still prescribed for pain relief, their role as antidepressants has largely been taken over by the serotonin reuptake inhibitors (SRIs).
Selective serotonin reuptake inhibitors (SSRIs)
These drugs inhibit the reuptake of serotonin but not of noradrenaline.
Examples:
| Generic name | Trade name |
| fluoxetine | Prozac® |
| paroxetine | Paxil® |
| sertraline | Zoloft® |
Although all these drugs quickly increase the amount of serotonin in the brain, there is more to the story than that. Unlike most psychoactive drugs, antidepressants do not relieve the symptoms of depression until a week or more after dosing begins. During this period, the number of serotonin receptors on the postsynaptic membranes decreases. How this translates into relief of symptoms is not yet understood.
Serotonin and norepinephrine reuptake inhibitors (SNRIs)
Because they act on the reuptake of both serotonin and noradrenaline (norepinephrine), this category of antidepressants is also known as dual reuptake inhibitors.
Examples: venlafaxine (Effexor®) and duloxetine (Cymbalta®).
Bupropion
Bupropion (Wellbutrin®) is a novel drug that blocks the reuptake of noradrenaline and dopamine. Although it does not interfere with the uptake of serotonin, it also appears to be an effective antidepressant.
Atomoxetine
This drug (Strattera®) selectively interferes with the reuptake of noradrenaline. It is used in children with attention deficit/hyperactivity disorder (ADHD).
Psychedelics
Psychedelic drugs distort sensory perceptions, especially sight and sound. Some such as mescaline, psilocybin and dimethyltryptamine (DMT) are natural plant products.

The photograph shows the peyote cactus in flower. The cactus head contains several psychedelic chemicals, of which mescaline is the most important. Dried cactus heads ("mescal buttons") have been used since pre-Columbian times in the religious ceremonies of native peoples in Mexico. About a century ago, this religious use spread to some tribes in the United States and Canada who, in 1922, became incorporated into the Native American Church. Other psychedelic drugs are synthetic. These include
- lysergic acid diethylamide (LSD)
- dimethoxymethylamphetamine (DOM or "STP")
- methylenedioxymethamphetamine (MDMA or "ecstasy")
As their name suggests, DOM and MDMA also share the stimulant qualities of amphetamines. All the psychedelics have a molecular structure that resembles serotonin and probably bind to serotonin receptors on the postsynaptic membrane.
Phencyclidine (PCP)
PCP is used as an anesthetic in veterinary medicine. Used (illicitly) by humans (called "crystal" or "angel dust"), it can produce a wide variety of powerful reactions resembling those of stimulants as well as psychedelics. Unlike the other psychedelics, it binds to (and inhibits) NMDA receptors (in the hippocampus and other parts of the forebrain).
Marijuana
The main psychoactive ingredient in marijuana is delta-9-tetrahydrocannabinol (Δ9-THC). It binds to
- CB1 receptors (G-protein-coupled receptors) that are present on presynaptic membranes in many parts of the brain.
- CB2 receptors are also found in the brain as well as being highly-expressed on cells of the immune system (e.g., B cells and T cells).
THC produces the drowsiness of sedatives like alcohol, the dulling of pain (like opioids) and in high doses, the perception-distorting effects of the psychedelics. Unlike sedatives and opioids, however, tolerance to THC does not occur. In fact, the drug is excreted so slowly from the body that, with repeated use, a given response is achieved by a lower dose.
The natural ligands of the CB receptors are the endocannabinoids - anandamide and 2-arachidonylglycerol (2-AG). Both of these compounds are produced from phospholipids.
What are these natural ligands doing? They probably will turn out to have multiple effects, but the clearest ones so far are their effects on
- appetite. Mice given anandamide eat more than normal while those whose genes for the CB1 receptor have been "knocked out" eat less than normal.These findings will be no surprise to the ill humans (e.g., with cancer or AIDS) who find that marijuana improves their appetite. Rimonabant (Acomplia®), a drug that blocks the ability of the body's natural CB1 ligands to bind the CB1 receptor was sold for a time in Europe as an appetite suppressant. (Because of its side effects, it was never approved for use in the U.S. and was removed from the European market in 2008.)
- development of correct synaptic connections in the embryonic brain. Mice whose genes for the CB1 receptor have been knocked out develop defects in the wiring pattern of interneurons in their brain (which may account for the cognitive defects that have been reported in the children of women who used marijuana during pregnancy).
- neuronal activity in the adult brain. Mice whose genes for the CB1 receptor have been knocked out are more susceptible to epileptic seizures. Marijuana has been used for centuries to control epileptic seizures in humans.
- suppressing contact dermatitis. Knockout mice lacking CB1 and CB2 receptors mount a more vigorous allergic inflammatory response to agents (like nickel) that elicit contact sensitivity.
Contributors and Attributions
John W. Kimball. This content is distributed under a Creative Commons Attribution 3.0 Unported (CC BY 3.0) license and made possible by funding from The Saylor Foundation.


