15.6.1: Human Hormones
- Page ID
- 5463
The essence of multicellularity is the coordinated interaction of the various kinds of cells that make up the body. Cells communicate with each other by chemical signals. Three kinds of chemical signaling can be distinguished:
- autocrine: the cell signals itself through a chemical that it synthesizes and then responds to. Autocrine signaling can occur solely within the cytoplasm of the cell or by a secreted chemical interacting with receptors on the surface of the same cell
- paracrine: chemical signals that diffuse into the area and interact with receptors on nearby cells. Examples include the release of cytokines that cause an inflammatory response in the area and the release of neurotransmitters at synapses in the nervous system.
- endocrine: the chemicals are secreted into the blood and carried by blood and tissue fluids to the cells they act upon.
This page will examine the properties of endocrine signaling.
Kinds of Hormones
There are two major classes of hormone: (1) proteins, peptides, and modified amino acids and (2) steroids.
Proteins, peptides and modified amino acids
These hydrophilic (and mostly large) hormone molecules bind to receptors on the surface of "target" cells; that is, cells able to respond to the presence of the hormone. These receptors are transmembrane proteins. Binding of the hormone to its receptor initiates a sequence of intracellular signals that may alter the behavior of the cell (such as by opening or closing membrane channels) or stimulate (or repress) gene expression in the nucleus by turning on (or off) the promoters and enhancers of the genes
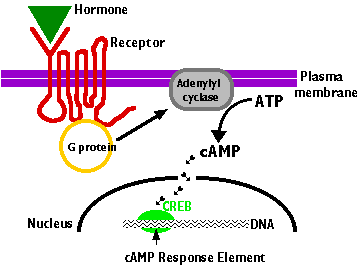
This is the sequence of events:
- The hormone binds to a site on the extracellular portion of the receptor.
- The receptors are transmembrane proteins that pass through the plasma membrane 7 times, with their N-terminal exposed at the exterior of the cell and their C-terminal projecting into the cytoplasm.
- Binding of the hormone to the receptor
- activates a G protein associated with the cytoplasmic C-terminal
- This initiates the production of a "second messenger". The most common of these are
- cyclic AMP, (cAMP) which is produced by adenylyl cyclase from ATP
- inositol 1,4,5-trisphosphate (IP3)
- The second messenger, in turn, initiates a series of intracellular events (shown here as short arrows) such as
- phosphorylation and activation of enzymes
- release of Ca2+ into the cytosol from stores within the endoplasmic reticulum
- In the case of cAMP, these enzymatic changes activate the transcription factor CREB (cAMP response element binding protein).
- Once bound to its response element 5' TGACGTCA 3' in the promoters of genes that are able to respond to the hormone, activated CREB turns on gene transcription.
- The cell begins to produce the appropriate gene products in response to the hormonal signal it had received at its surface.
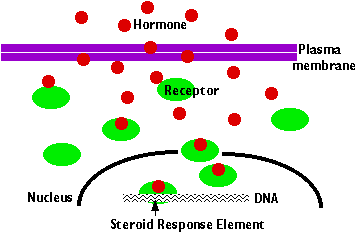
Steroid Hormones
Steroid hormones, being hydrophobic molecules, diffuse freely into all cells. However, their "target" cells contain cytoplasmic and/or nuclear proteins that serve as receptors of the hormone. The hormone binds to the receptor and the complex binds to hormone response elements — stretches of DNA within the promoters of genes responsive to the hormone. The hormone/receptor complex acts as a transcription factor turning target genes "on" (or "off").
Hormone Regulation
The levels of hormones circulating in the blood are tightly controlled by three homeostatic mechanisms:
- When one hormone stimulates the production of a second, the second suppresses the production of the first. Example: The follicle stimulating hormone (FSH) stimulates the release of estrogens from the ovarian follicle. A high level of estrogen, in turn, suppresses the further production of FSH.
- Antagonistic pairs of hormones. Example: Insulin causes the level of blood sugar (glucose) to drop when it has risen. Glucagon causes it to rise when it has fallen.
- Hormone secretion is increased (or decreased) by the same substance whose level is decreased (or increased) by the hormone. Example: a rising level of Ca2+ in the blood suppresses the production of the parathyroid hormone (PTH). A low level of Ca2+ stimulates it.
Hormone Transport
Although a few hormones circulate simply dissolved in the blood, most are carried in the blood bound to plasma proteins. For example, all the steroid hormones, being highly hydrophobic, are transported bound to plasma proteins.
| Hormone | Structure (1) | Principal Source |
|---|---|---|
| Thyroid-stimulating hormone (TSH) | protein (201) | Anterior lobe of pituitary |
| Follicle-stimulating hormone (FSH) | protein (204) | |
| Luteinizing hormone (LH) | protein (204) | |
| Prolactin (PRL) | protein (198) | |
| Growth hormone (GH) | protein (191) | |
| Adrenocorticotropic hormone (ACTH) | peptide (39) | |
| Vasopressin | peptide (9) | Posterior lobe of pituitary |
| Oxytocin | peptide (9) | |
| Thyrotropin-releasing hormone (TRH) | peptide (3) | Hypothalamus |
| Gonadotropin-releasing hormone (GnRH) | peptide (10) | |
| Growth hormone-releasing hormone (GHRH) | peptides (40, 44) | |
| Corticotropin-releasing hormone (CRH) | peptide (41) | |
| Somatostatin | peptides (14, 28) | |
| Dopamine | tyrosine derivative | |
| Melatonin | tryptophan derivative | Pineal gland |
| Thyroxine (T4) | tyrosine derivative | Thyroid Gland |
| Calcitonin | peptide (32) | |
| Parathyroid hormone (PTH) | protein (84) | Parathyroid glands |
| protein (251) | Bone | |
| Osteocalcin | peptide (49) | |
| Erythropoietin (EPO) | protein (166) | |
| Glucocorticoids (e.g., cortisol) | steroids | Adrenal cortex |
| Mineralocorticoids (e.g., aldosterone) | steroids | |
| Androgens (e.g., testosterone) | steroids | |
| Adrenaline (epinephrine) | tyrosine derivative | Adrenal medulla |
| Noradrenaline (norepinephrine) | tyrosine derivative | |
| Estrogens (e.g., estradiol) | steroid | Ovarian follicle |
| Progesterone | steroid | Corpus luteum and placenta |
| Human chorionic gonadotropin (HCG) | protein (237) | Trophoblast and placenta |
| Androgens (e.g., testosterone) | steroid | Testes |
| Insulin | protein (51) | Pancreas (Islets of Langerhans) |
| Glucagon | peptide (29) | |
| Somatostatin | peptides (14, 28) | |
| Amylin | peptide (37) | |
| Erythropoietin (EPO) | protein (166) | Kidney |
| Calcitriol | steroid derivative | |
| Calciferol (vitamin D3) | steroid derivative | Skin |
| Atrial-natriuretic peptide (ANP) | peptides (28, 32) | Heart |
| Gastrin | peptides (e.g., 14) | Stomach and intestine |
| Secretin | peptide (27) | |
| Cholecystokinin (CCK) | peptides (e.g., 8) | |
| Fibroblast Growth Factor 19 (FGF19) | protein (216) | |
| Incretins | peptides (e.g., 31, 42) | |
| Somatostatin | peptides (14, 28) | |
| Neuropeptide Y | peptide (36) | |
| Ghrelin | peptide (28) | |
| PYY3-36 | peptide (34) | |
| Serotonin | tryptophan derivative | |
| protein (70) | Liver | |
| Angiotensinogen | protein (485) | |
| Thrombopoietin | protein (332) | |
| Hepcidin | peptide (25) | |
| Betatrophin | protein (193) | |
| Leptin | protein (167) | Fat cells (adipocytes) |
| Retinol Binding Protein 4 | protein (~180) | |
| Adiponectin | protein (117) | |
| Asprosin | protein (140) |
Note: Numbers within parentheses indicate the number of amino acids in the protein or peptide(s).


