15.4V: AIDS
- Page ID
- 5458
AIDS stands for Acquired Immune Deficiency Syndrome. It represents the late stages of infection by a retrovirus called Human Immunodeficiency Virus (HIV). The immune deficiency is caused by the loss of the CD4+ T cells that are essential for both cell-mediated immunity and antibody-mediated immunity.
HIV - Human Immunodeficiency Viruses
They are retroviruses. There are two of them:
- HIV-1 — the major cause of AIDS throughout the world;
- HIV-2 — mostly found in West Africa.
Infection
HIV can only enter cells that express
- the transmembrane protein CD4 found on helper T cells
- a second G protein-coupled "coreceptor" (GPCR) on these cells:
- Strains of HIV (designated "R5") bind the coreceptor CCR5. These are the strains that are most infectious.
- Strains of HIV (designated "X4") bind the coreceptor CXCR4.
- Both strains usually coexist in an ongoing infection with X4 tending to dominate in the final stages of AIDS.

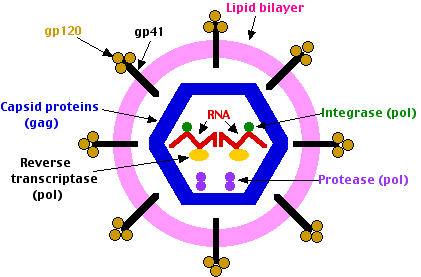
The virion binds to both CD4 and either coreceptor by means of molecules on its surface called glycoprotein 120 (gp120). The virion then is swept into the cell by receptor-mediated endocytosis. Fusion of the lipid membranes of the virion and the endosome - Endocytic vesicle, releases the contents of the virion into the cytosol. The fusion is mediated by gp41. When HIV infects a cell its molecules of reverse transcriptase and integrase are carried into the cell attached to the viral RNA molecules. The reverse transcriptase synthesizes DNA copies of the RNA. These enter the nucleus where the integrase catalyzes their insertion into the DNA of the host's chromosomes. The HIV DNA is transcribed into fresh RNA molecules which reenter the cytosol where some are translated by host ribosomes. The env RNA is translated into molecules of the envelope protein (gp160). These pass through the endoplasmic reticulum and then the Golgi apparatus where they become glycosylated by enzymes of the host cell.
Proteases of the host cell then cut gp160 into
- gp120 which sits on the surface of the virions (and is the target of most of the vaccines currently being tested).
- gp41, a transmembrane protein associated with gp120.
- the gag and pol genes are translated into a single protein molecule which is cleaved by the viral protease into
- 6 different capsid proteins
- the protease
- reverse transcriptase
- the integrase
- other RNA molecules become incorporated into fresh virus particles
Disease Transmission
HIV is present in body fluids especially blood and semen, especially in the early and late phases of the disease. Breaks or abrasions in mucous membranes and skin allow the virus in.
In North America, transmission occurs primarily
- between men when one ejaculates into the rectum (or mouth — the adenoids and tonsils are filled with dendritic cells) of the other
- among intravenous drug users who share needles
- in women who are the sexual partners of bisexual men or i.v. drug users
- in the newborn babies of these women
- in recipients of infected blood or blood products. This last category accounted for a devastating epidemic among hemophiliacs in the 1980s who unknowingly used HIV-contaminated preparations of factor 8 (VIII). In some areas, 90% or more of the hemophiliacs developed AIDS. That risk, and the risk from blood transfusions, is now virtually zero because
- all donated blood is now tested to see if the donor has been infected with HIV (as well as some other viruses)
- plasma-derived preparations of factors 8 (VIII) and 9 (IX) are now treated with heat and/or solvents to destroy any viruses that might be present;
- recombinant factor 8 (VIII) and recombinant factor 9 (IX) made by genetic engineering are now available.
Disease Progression
Infection by HIV produces three phases of disease:
- an early phase that
- lasts about 2 weeks
- is accompanied by fever, aches, and other flu-like symptoms
- is accompanied by high levels of virus in the blood.
- a middle phase with these features:
- lasts for months or even years
- produces few, if any, symptoms
- patient's blood contains few viruses, but contains antibodies to the virus which are the basis of the most common test for HIV infection
- continuous infection, death, and replacement of CD4+ T cells
- It is the late phase that is called AIDS. It has these features:
- A rapid decline in the number of CD4+ T cells. When these drop below about 350 per µl (normal is >1000), the patient's immunity is sufficiently weakened that opportunistic infections begin. These are infections caused by organisms that ordinarily do not cause disease symptoms in immunocompetent people. They include:
- viruses, e.g., herpes simplex, herpes varicella-zoster, Epstein-Barr virus (EBV)
- bacteria, e.g., Mycobacterium tuberculosis
- fungi, e.g. Candida albicans (the cause of "thrush"), Pneumocystis jirovecii (causes pneumonia)
- protozoans, e.g., Microsporidia
- When the CD4+ count drops below 200 per µl (mm3), opportunistic infections become more severe and cancer (e.g., lymphoma, Kaposi's sarcoma) may develop. Untreated, these usually kill the patient within a year or so.
- A rapid decline in the number of CD4+ T cells. When these drop below about 350 per µl (normal is >1000), the patient's immunity is sufficiently weakened that opportunistic infections begin. These are infections caused by organisms that ordinarily do not cause disease symptoms in immunocompetent people. They include:
Treatment
In affluent countries, the progression of HIV disease has been markedly slowed by the use of HAART (= Highly Active AntiRetroviral Therapy). This refers to combined therapy with three or more drugs, e.g., two that target the reverse transcriptase and one that targets the viral protease.
Reverse Transcriptase Inhibitors
- Nucleoside analogs. Examples:
- zidovudine (AZT)(Retrovir®)
- lamivudine (Epivir®)
- didanosine (Videx®)
Each of these drugs "fools" the reverse transcriptase into incorporating it into the growing DNA strand which then halts further DNA synthesis.
- Other Reverse Transcriptase Inhibitors
- These drugs, e.g., efavirenz (Sustiva®) inhibit the enzyme by other mechanisms.
Protease Inhibitors
These block the viral protease so that the proteins needed for assembly of new viruses cannot be cleaved from the large protein precursor. Examples:
- indinavir (Crixivan®)
- saquinavir (Invirase®)
- ritonavir (Norvir®)
Fusion Inhibitors
Fusion of the virion membrane with the endosome membrane involves noncovalent binding between two segments of the gp41 molecule designated HR1 and HR2. Enfuvirtide (Fuzeon®), a synthetic polypeptide containing 36 of the amino acids present in the HR2 segment, interferes with this process. It probably acts as a kind of competitive inhibitor, binding to HR1 thus preventing HR2 from binding HR1.
Integrase Inhibitors
Raltegravir (Isentress®), a drug that inhibits the HIV-1 integrase, has slowed disease progression in patients for whom other drugs were losing their effectiveness.
Inhibiting Coreceptor Binding
Several drugs — as well as some monoclonal antibodies — that block the binding of HIV to the coreceptors CCR5 and CXCR4 are being tested for safety and efficacy. Maraviroc®, a drug that binds to CCR5, has performed so well that it received FDA approval in 2007. People who have a mutation in their CCR5 gene are resistant to infection. Early clinical trials of gene therapy in which a patient's normal CCR5 gene is deliberately disrupted have shown promise.
Problems with drug treatment
Despite the great advances in slowing the progression of the disease, reversing at least for a time the symptoms of the late stages of the disease and preventing the infection of babies born to infected mothers drug therapy has many drawbacks.
- The drugs are so expensive ($7,000 to $10,000 per year) that they not only drain resources in affluent countries but are simply unavailable in the many poor countries where the epidemic rages.
- They have many unpleasant side-effects (e.g., nausea, diarrhea, liver damage).
- They demand a very complicated dosing regimen: over a dozen pills a day (not counting those needed to cope with the accompanying opportunistic infections).
- They have to be continued even after active virus disappears because HIV-1 can integrate into the DNA of resting memory CD4+ T cells as a provirus and emerge as active virus later.
- They often lose effectiveness as they select for the emergence of drug-resistant virions in the patient. This latter problem is particularly serious because of the speed at which mutations occur in HIV (as we shall now see).
Genetic Variability of HIV
Reverse transcription (RNA → DNA) lacks the proofreading capabilities of DNA replication or of normal transcription (DNA → RNA). Therefore errors, i.e., mutations, are frequent. Because of these,
- The population of viruses in a single patient becomes genetically more diverse as time goes by. This can lead to:
- appearance of strains that invade other types of cells such as X4 strains that target T cells and strains that target cells of the brain, etc.
- Development of resistance to the anti-viral drugs being used.
- New strains and subtypes of HIV-1 and HIV-2 arise in the human population.
- These complicate the efforts to develop a vaccine against HIV
- But as we shall now see that these have helped to unravel the origins of the disease.
Origin of HIV
Genome sequencing of different isolates of HIV-1 and HIV-2 shows that each is related to retroviruses that occur in primates in Africa. These are designated simian immunodeficiency viruses (SIV) although they do not cause immune deficiency (or any disease) in their natural host. However, on those occasions when a SIV accidentally infects a primate of a different species, it does cause disease in the new host. The human epidemic is one example.
- HIV-1 is most closely related to a SIV found in chimpanzees (Pan troglodytes troglodytes)
- HIV-2 is most closely related to a SIV that occurs in the sooty mangabey (Cercocebus atys).
Genome analysis also permits the construction of phylogenetic trees which reveal different clades of HIV just as such analysis reveals evolutionary relationship between species. The picture so far:
- HIV-1 appears to have infected humans on at least 4 different occasions giving rise to 4 clades: M, N, O, and P. Groups M and N appear to have jumped at separate times from chimpanzees to humans while O may have jumped from gorillas to humans. Except in parts of West Africa, most human cases are caused by members of Group M.
- HIV-2 appears to have jumped from sooty mangabeys to humans on at least 4 different occasions (there are 4 clades).
- How? These (and other) primates are often slaughtered for food and exposure to their blood and tissues is probably the route of transmission. In fact the chimpanzee SIV that gave rise to HIV-1 appears to be itself the product of recombination between two monkey SIVs that infected chimpanzees. (Chimps often eat monkeys.)
Just as with other evolutionary trees, one can also estimate from genome sequences the time of divergence of two branches. This evidence indicates that the Group M clade of HIV-1 invaded humans sometime very early in the 20th century.
But the worldwide epidemic of AIDS did not get its start until the 1980s. What took so long? An answer to that requires an appreciation of the way in which contagious diseases spread. Their rate of spread depends on:
- The ease of transmission. The transmissibility of HIV is very low. HIV is not like influenza or measles which spread like wildfire.
- The length of time the host remains contagious. Again, HIV is not like influenza or measles where the period of contagiousness is just a few days. For HIV, it can be years.
- The number of susceptible contacts; that is, the proximity of potential new hosts. For sexually-transmitted diseases (STDs), that means the number of sexual contacts.
So diseases like HIV only smolder in isolated populations because they lack the density of susceptible contacts. In crowded populations, the equation changes. There has been a dramatic population shift from rural to urban areas in sub-Saharan Africa since 1950. In the case of STDs, the availability of multiple sexual contacts — perhaps accompanied by changing sexual mores — tips the scales. In any case, the major factor today in the spread of HIV is promiscuity, whether homosexual or heterosexual.
Prevention of AIDS
Vaccines
Many once-feared infectious diseases have been reduced or eliminated by the development of a vaccine to prevent the disease. Over two dozen experimental anti-HIV vaccines have been developed and clinical trials of some of these have been and are presently being undertaken. So far, the results have been disappointing. There are probably several reasons. Some of the vaccines attempt to induce antibodies, e.g., against the outer portion of the envelope protein (called gp120). But antibody-mediated immunity may not give adequate protection. The gene (env) encoding the envelope protein mutates too rapidly. The virus may be able to stay within cells out of the reach of circulating antibodies. High levels of antibodies (the basis of the most common test of infection) persist even while the disease pursues its inexorable course.
So other vaccines have been designed to favor the development of cell-mediated immunity; e.g., cytotoxic T cells.
Many of these are DNA vaccines, for example,
- a live virus such as canarypox (a harmless relative of smallpox) or an adenovirus which serves as a vector for introducing HIV genes (DNA)
- a mixture of plasmids encoding several HIV genes (e.g., gag, pol, and env of several HIV strains).
It is hoped that expression of these genes within the cells (e.g., muscle) of the subject will induce a protective immune response, but to date there has been no success.
Behavior
Because HIV transmission is so difficult, changing behavior could go a long way toward stopping the epidemic.
- Reducing the number of sexual partners.
- If injecting drugs cannot be stopped, then using sterile needles (thus not sharing them) would prevent infection.
- Using condoms and/or other (e.g., chemical) barriers to prevent contact with infectious semen.
In the words of Anthony S. Fauci, Director of the National Institute of Allergy and Infectious Diseases, "Unlike microbial scourges, such as malaria and tuberculosis (among many others), for which there is very little that people can do to prevent infection, HIV infection in adults is entirely preventable by behavior modification".
Contributors and Attributions
John W. Kimball. This content is distributed under a Creative Commons Attribution 3.0 Unported (CC BY 3.0) license and made possible by funding from The Saylor Foundation.


