2.2: Bacterial Growth and Reproduction
- Page ID
- 18127
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Flagella and motility
- monotrichous flagella - the bacterial cell has a single flagella
- peritrichous flagella - the bacterial cell has several flagella which are located at various sites on the cell surface (e.g. E. coli)
Motility is due to the presence of one or more flagella.
- in peritrichous flagellate bacteria the flagella rotate independently of one another
- 95% of the time the flagella rotate counterclockwise
- 5% of the time the flagella switch directions and rotate clockwise
- When the flagella are all rotating counterclockwise, the flagella are bundled together and the bacteria travels in a straight line (i.e. it swims)
- When one flagella switches direction the bundle disassociates, and the bacteria tumbles
- alternative swimming and tumbling results in a three-dimensional random walk
- chemoattractants and repellents can interaction with receptor proteins in the cell envelope, which in turn influence the rate of tumbling when the cell is moving in a given direction
Growth and Reproduction
Essential requirements for growth include:
- supply of suitable nutrients
- source of energy
- water
- appropriate temperature
- appropriate pH
- appropriate levels (or absence) of oxygen
Nutrients
Cells need a source of:
- carbon
- nitrogen
- phosphorous
- sulfur
- other trace materials
Although a given bacteria typically uses a limited range of compounds, bacteria as a group can utilize a wide range of compounds as nutrients:
- sugars and carbohydrates
- amino acids
- sterols
- alcohols
- hydrocarbons
- methane
- inorganic salts
- carbon dioxide
Energy
Energy is needed for
- essential chemical reactions
- uptake of nutrients
- flagellar motility
Phototrophic vs chemotrophic bacteria
- phototrophic - energy derived from light source
- chemotrophic - energy is obtained by processing chemicals from the environment
Water
- 80% of the mass of typical bacteria is water
- Water is needed for growth and reproduction
- Dessication (extreme lack of water) is tolerated to different degrees by different bacteria
Temperature
- Growth proceeds most rapidly at the optimum growth temperature for a particular bacteria (and decreases as temperature is raised or lowered from this optimum)
- For any bacteria, there is a minimum and maximum temperature beyond which growth is not supported
Thermophilic bacteria
- optimum growth temperature is >45°C
- occur in compost piles, hot springs and ocean floor hydrothermal vents
- Pyrodictium have an optimum growth temperature of 105°C
Mesophilic bacteria
- optimum growth temperatures between 15 and 45°C
- live in a wide range of habitats
- since the human body is 37-42°C, human pathogenic bacteria are mesophiles
Psychrophilic bacteria
- optimum at 15°C or below
- minimum temperature of 0°C, or less
- maximum temperature of 20°C
- occur in polar seas
pH
- most bacteria grow optimally near neutral pH (7.0)
- acidophiles have an optimum pH with is more acidic (Thermoplasma acidophilum, found in hot springs, prefers pH 0.8-3, and will not grow at neutral pH)
- alkalophiles have an optimum at higher (alkaline) pH ranges (Exiguobacterium aurantiacum, found in natural alkaline lakes, prefers pH 8.5-9.5)
Oxygen
- bacteria which must have oxygen for growth are termed obligate aerobes
- bacteria which can grow only in the absence of oxygen are termed obligate anaerobes (e.g. environment which are isolated from the atmosphere; e.g. river mud, and within the intestines, for example)
- bacteria which normally grow in the presence of oxygen, but which can manage to grow in its absence, are termed faculative anaerobes
- conversely, bacteria which normally grow anaerobically, but which can manage to grow in the presence of oxygen, are termed faculative aerobes
Inorganic ions
All bacteria need low concentrations of certain inorganic ions in order to functions, e.g.
- iron for cytochromes (energy metabolism), and certain enzymes
- magnesium for cell wall stability
- manganese and nickel in metabolic enzymes
- high concentrations usually inhibit growth (e.g. salt has been used in the preservation of pork, beef and cod)
- some bacteria, halophiles, grow only in the presence of high concentrations of certain salts (e.g. sodium chloride). Halobacteriaceae grow only in the presence of 3-4 M NaCl. This amount of salt is needed to maintain the structure of the cell wall and internal molecular assemblies (e.g. ribosomes).
Growth in a single cell (e.g. Escherichia coli - a gram negative bacillus)
The cycle of events in which a cell grows, and divides into two daughter cells, is called the cell cycle.
"Slow Growth"
.png?revision=1&size=bestfit&width=502&height=371)
Figure 2.2.1: Replication for slow growth
- Replication begins at a specific place on the chromosome - the origin or "ori" region.
- During "slow growth" each new daughter cell contains exactly one chromosome, because a new round of chromosomal replication does not begin until after completion of cell division
The cell division cycle can be thought of as a linear sequence of three periods: I, C and D
- C is the period during which chromosomal replication occurs
- D is the period in which the septum forms, and cell division occurs at the end of the D period
- I is the period between each successive initiation of chromosomal replication
For the above type of slow growth, the relationship between these three periods is as follows:
.png?revision=1&size=bestfit&width=637&height=130)
Figure 2.2.2: Periods of cell cycle for slow growth
- I is also known as the doubling time of the bacterial growth
"Rapid Growth"
.png?revision=1&size=bestfit&width=401&height=426)
Figure 2.2.3: Replication for rapid growth
- A new round of chromosome replication begins before cell division occurs
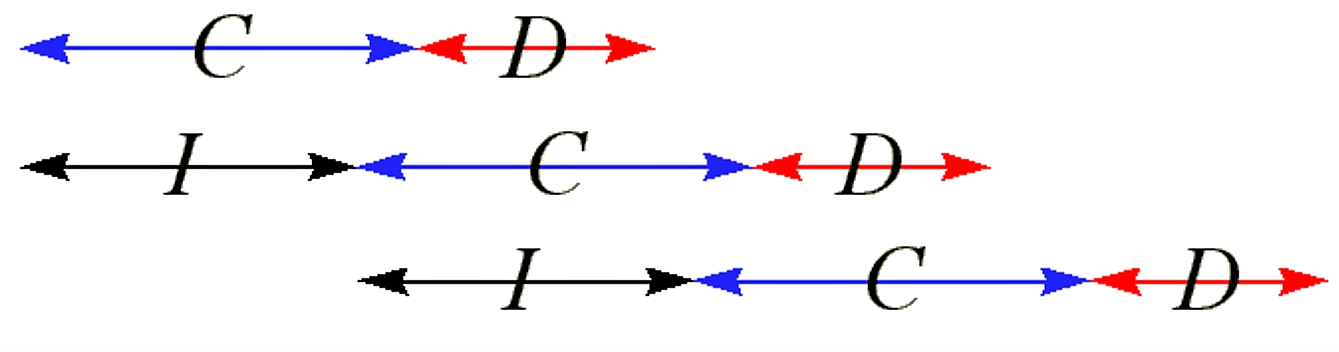.png?revision=1&size=bestfit&width=529&height=139)
Figure 2.2.4: Cell cycle for rapid growth
- Each daughter cell has the equivalent of about 1 ½ chromosomes
- In rapidly growing E. coli cells the C period is about 42 minutes and D is about 25 minutes.
- The maximum doubling time for E. coli is about 20 minutes
- "medium": any solid or liquid specially prepared for bacterial growth
- "culture": a liquid or solid medium containing bacteria which have grown (or are growing) in or on that medium
- "incubation": the process of maintaining a particular temperature (and/or other desirable conditions) for bacterial growth
- "innoculation": the initial process of adding the cells to the medium
Growth on a solid medium
- Liquid solution of nutrients plus 1% agar (the preferred form of Jello in Asia. A polysaccharide extracted from seaweed; as opposed to Western Jello which is a protein extracted from the hooves of large farm animals) forms solid media
Typical Solid Media Recipe (1 liter). The media here is commonly called "Luria Broth", or "LB". It is named after one of the scientists who developed it (not "Dr. Broth").
- Yeast extract 5 g (nucleic acids, cofactors, inorganic salts, carbohydrates)
- Tryptic digest of casein (milk protein) 10g (peptides and amino acids)
- NaCl 10g (note: final concentration is thus 0.17 M, or close to physiological)
- Agar 10g
- Water (bring volume up to 1.0 liter)
- Autoclave, pour into petri dish, let cool
An individual bacterial cell will divide and eventually become a visible mass of cells known as a colony
If instead of a single cell, the solid media is initially populated with a large number of cells, confluent growth or a lawn of bacteria will be visible
.png?revision=1&size=bestfit&width=400&height=365)
Figure 2.2.5: Growth on solid medium
"Streaking" solid media plates
- bacteria can be introduced onto solid media by a sterile transfer tool, such as a wire loop (nichrome wire), or autoclaved toothpick, which has been dipped into a bacterial culture
- such a transfer contains thousands of individual bacterial cells
- it is desirable to grow colonies from individual cells rather than from a large population
This is done to avoid the takeover of the strain by potential wild-type revertants
- "streaking" is a method to isolate individual cells for growth on solid media from an inoculation which originally contains thousands of cells
.png?revision=1&size=bestfit&width=489&height=467)
Figure 2.2.6: Streaking
Growth in liquid medium
- No agar
- Use Erlenmeyer flask, or fermenter
- If necessary, aerate, agitate
- progeny will be dispersed throughout medium (diffusion or locomotion)
- as the cell density increases, the media becomes turbid
- number of cells plotted versus time will yield a growth curve
.png?revision=1&size=bestfit&width=507&height=483)
Figure 2.2.7: Growth curve in liquid medium
- "lag phase" after inoculation cells are becoming acclimated to the new environment (temp, nutrients, etc.)
- "log phase" cells have adapted and are dividing at a constant rate (i.e. the maximum for the species under the given conditions of temp, pH, nutrients, oxygen, etc.)
- "stationary phase" cell growth ceases as nutrients are exhausted and/or waste products build up in the media
- "death phase" number of viable (living cells) in the stationary phase culture decreases (usually due to toxicity of waste products)
Cell density can be conveniently monitored using the absorbance of visible light (usually at 600 nm)
.png?revision=1&size=bestfit&width=463&height=381)
Figure 2.2.8: Cell density curve
Different media will result in different growth rates and different stationary phase densities
- Rich media will have short (<1.0 hour) doubling times and will result in higher cell densities at stationary phase
- Minimal media will exhibit slow growth (doubling times ~1.0 hour at 37°C) and low final densities
- Efficient agitation and aeration can increase final cell densities (fermenters may achieve higher densities than shaker flasks).
|
Minimal |
LB |
Terrific |
|
|
Phosphate salts |
16g |
- |
12g |
|
Ammonium salts |
1g |
- |
- |
|
Magnesium salts |
0.1g |
- |
- |
|
Glucose/glycerol |
4g |
- |
4g |
|
Sodium Chloride |
0.5g |
10g |
- |
|
Enzymatic digest of casein (milk protein) |
- |
10g |
12g |
|
Yeast extract |
- |
5g |
24g |
|
Approx doubling time (min) |
60 |
45 |
30 |
|
Stationary phase density (A600) |
3 |
7 |
15 |
Let's take a look at some raw data from a culture of E. coli growing in a fermenter. The absorbance at 600nm was recorded at various time intervals:
|
Time |
A600 |
|
0 |
0.09 |
|
18 |
0.102 |
|
78 |
0.124 |
|
142 |
0.253 |
|
205 |
0.487 |
|
255 |
1.02 |
|
322 |
1.98 |
|
378 |
3.95 |
|
446 |
5.88 |
|
504 |
6.76 |
|
564 |
7.2 |
`
The growth curve looks like this:
.png?revision=1&size=bestfit&width=529&height=365)
Figure 2.2.9: Sample growth curve
In order to calculate the doubling time we need to know the region of the growth curve for which the growth is logarithmic. We can evaluate this by plotting the absorbance data on a logarithmic (log10) scale. In this case, our data will look like this:
|
Time |
A600 |
log10 |
|
0 |
0.09 |
-1.05 |
|
18 |
0.102 |
-0.99 |
|
78 |
0.124 |
-0.91 |
|
142 |
0.253 |
-0.60 |
|
205 |
0.487 |
-0.31 |
|
255 |
1.02 |
0.01 |
|
322 |
1.98 |
0.30 |
|
378 |
3.95 |
0.60 |
|
446 |
5.88 |
0.77 |
|
504 |
6.76 |
0.83 |
|
564 |
7.2 |
0.86 |
.png?revision=1&size=bestfit&width=550&height=424)
Figure 2.2.10: Sample logarithmic growth curve
In this case, the data appears to have an initial lag period followed by logarithmic growth until about 400 minutes, then the rate slows. In other words, the log10 curve appears linear over the time period 100 - 400 minutes, or so.
We can conveniently determine the doubling time by replotting the data over this period and converting the A600 into log2 values.
Note
logN(X) = log10(X)/log10(N)
|
Time |
A600 |
log10 |
log2 |
|
0 |
0.09 |
-1.05 |
|
|
18 |
0.102 |
-0.99 |
|
|
78 |
0.124 |
-0.91 |
-3.01 |
|
142 |
0.253 |
-0.60 |
-1.98 |
|
205 |
0.487 |
-0.31 |
-1.04 |
|
255 |
1.02 |
0.01 |
0.029 |
|
322 |
1.98 |
0.30 |
0.99 |
|
378 |
3.95 |
0.60 |
1.98 |
|
446 |
5.88 |
0.77 |
|
|
504 |
6.76 |
0.83 |
|
|
564 |
7.2 |
0.86 |
.png?revision=1&size=bestfit&width=503&height=394)
Figure 2.2.11: Sample log2 growth curve
If we plot a straight line through these points the slope will give us the rate of change of the log2 A600 values as a function of time:
.png?revision=1&size=bestfit&width=443&height=356)
Figure 2.2.12: Linear fit for sample log2 growth curve
- Thus, for this culture, the growth (slope) can be described as a rate of 0.0167 log2A600/min.
- The inverse of the slope will therefore tell us how many minutes it takes for the culture to increase its density by 1 log2A600 absorbance units.
- Since it is log2, a change of 1 absorbance units means the absorbance has doubled.
Therefore, the inverse of the slope for the log2 plot gives us the time it takes for the culture absorbance to double (i.e. the doubling time)
For this experiment, the doubling time is 1/0.0167 or 59.9 minutes. This suggests that the media is probably not very rich (maybe like minimal media), however, the final absorbance is higher than that expected for minimal media. So, possibly the media LB, but the growth was done at a lower temperature (i.e. leading to a slower growth rate).
Important parameters to determine:
- A600 at stationary phase
- A600 at ½ stationary phase (usually the time point chosen to stimulate bacteria to produce recombinant proteins)
- Doubling time (plot log2 of A600 over logarithmic range of growth, take inverse slope)


