1.8: pH
- Page ID
- 3748
pH is a measure of the concentration of hydrogens ions (= H+) (= protons) in a solution. Numerically it is the negative logarithm of that concentration expressed in moles per liter (M).
Pure water spontaneously dissociates into ions, forming a 10-7 M solution of H+ (and OH-). The negative of this logarithm is 7, so the pH of pure water is 7.
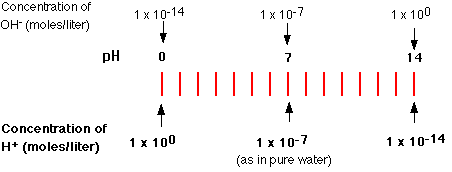
Solutions with a higher concentration of H+ than occurs in pure water have pH values below 7 and are acidic. Solutions containing molecules or ions that reduce the concentration of H+ below that of pure water have pH values above 7 and are basic or alkaline.
Is pH important? Yes!
The properties of most proteins, enzymes for example, are sensitive to pH.
As the pH drops,
- H+ bind to the carboxyl groups (COO-) of aspartic acid (Asp) and glutamic acid (Glu), neutralizing their negative charge, and
- H+ bind to the unoccupied pair of electrons on the N atom of the amino (NH2 ) groups of lysine (Lys) and arginine (Arg) giving them a positive charge.
The result: Not only does the net charge on the molecule change (it becomes more positive) but many of the opportunities that its R groups have for ionic interactions with other molecules and ions are altered.
As the pH rises,
- H+ are removed from the COOH groups of Asp and Glu, giving them a negative charge (COO-), and
- H+ are removed from the NH3+ groups of Lys and Arg removing their positive charge.
The result: Again the net charge on the molecule changes (it becomes more negative) and, again, many of the opportunities its R groups have for ionic interactions with other molecules or ions are altered.
The pH of the cytosol within a human cell is about 7.4. BUT, this value masks the pH differences that are found in various compartments within the cell. For example,
- The interior of lysosomes is much more acidic (as low as pH 4) than the cytosol, and the enzymes within work best at these low pH values.
- The pH differential created within chloroplasts by the energy of the sun is harnessed to synthesize ATP which, in turn, powers the synthesis of food.
- The pH differential created within mitochondria during the respiration of food is harnessed to the synthesis of ATP which, in turn, powers most of the energy-consuming activities of the cell such as locomotion and biosynthesis of cell components.


