Storylab: Diffusion
- Page ID
- 27810
This page is a draft and is under active development.
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)How Can Diffusion Be Observed?
by Shannan Muskopf, biologycorner.com
 When I got to class today, there was a bag on my desk with a milky white liquid inside and a note on the board that said “Investigation: How Can Diffusion be Observed?” Underneath that bold statement, was a request to check the bag on the desk for leaks but be careful to not break it. It looked like one of those cheap sandwich bags and a knot was tied in the top. The white stuff wasn’t all the way mixed and some had settled to the bottom of the back, it looked thick and goopy. What was in this bag?
When I got to class today, there was a bag on my desk with a milky white liquid inside and a note on the board that said “Investigation: How Can Diffusion be Observed?” Underneath that bold statement, was a request to check the bag on the desk for leaks but be careful to not break it. It looked like one of those cheap sandwich bags and a knot was tied in the top. The white stuff wasn’t all the way mixed and some had settled to the bottom of the back, it looked thick and goopy. What was in this bag?
The teacher arrived to class and asked us if we had examined the bags for leaks. Everyone in the class confirmed that none of the stuff was leaking out of their bag. “Good,” said Ms. Hooke, “because that could really ruin the experiment. We wouldn’t want to have leaky bags.”
She then told us that the bags were going to be models for the cell. The plastic of the bag represented the cell membrane, and the white stuff was like the cell’s cytoplasm. “Like the cell membrane, the plastic of the bag is semipermeable.” Ms. Hooke paused and stared at Bessie, who looked like she was asleep. Uh-oh. Ms. Hooke pounced on Bessie and dropped a plastic bag in front of her which made a loud “splat” on the desk, but it didn’t break. “Bessie! Do you know what it means to be semipermeable.”
Bessie was startled and sat up quickly, and I almost thought she was going to fall out of her chair. “Semi-what?”
Ms. Hooke sighed and pointed at Bessie. “Best pay attention. Semipermeable means that some stuff can get through the bag, but not everything. You see, the molecules of the plastic are like a mesh or a fence. If molecules are small enough, they can get through. Imagine a screen on a window. Air can get through, but mosquitoes cannot. That’s what this bag is like. It feels solid to us, and isn’t leaking, but if you were Ant-Man, and you were small enough, you could fit between the molecules.”
Ms. H pulled out a box that said corn starch. “I bought this at the store, you can use it to make gravy and other ...stuff. Today, I used it to make a cell.” She jiggled the bag filled with corn start and I was worried for a second that she might throw it at Bessie.
“Do you think the bag is permeable to the starch and water solution?” She looked around, and I could practically see Bessie squirming, so I decided to be nice and raise my hand.
“It’s not permeable, because if it was, we would have seen the water or the white stuff leak out when we were playing with it.” Ms. H gave an approving nod to my answer.
 Next, she pulled out a beaker that had some orange stuff in it. “Behold!” she said dramatically. “A beaker full of iodine!”
Next, she pulled out a beaker that had some orange stuff in it. “Behold!” she said dramatically. “A beaker full of iodine!”
The class didn’t seem too impressed, but you never knew what was going to happen in this class. “So, what’s really cool about iodine, is that it’s an indicator. Indicators are chemicals that let us know if another substance is present, usually by turning colors.” At that, she tilted the box of cornstarch into a beaker full of water. The water turned a milky white color, like the water in the bag. She held the beaker up to the class. “Now watch this.”
I leaned in, thinking something amazing was going to happen. Ms. H took poured the iodine into the beaker full of cornstarch. Where the iodine hit the solution, it turned from white to a dark purple.
“Did you see that!” Ms. H seemed super-excited, and I guess it was cool, but not that exciting. “Whenever iodine comes into contact with starch, it turns purple.” Then she did do something shocking, she grabbed Bessie’s paper and splashed some iodine on it, every spot that the liquid touched also turned purple. What?!
There was a dramatic pause as Ms. H held up the speckled paper. “Class… what’s in this paper?” It seemed like the class got it all at once and shouted out the answer: “Cornstarch!”
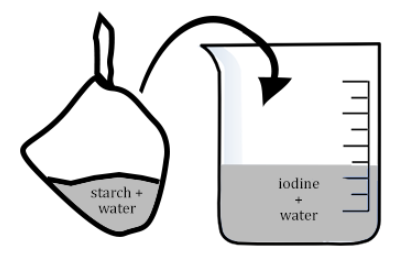
Ms. Hooke then asked Bessie to put a beaker of iodine on each of the lab tables. Now that everyone had a bag and a beaker, I had a feeling I knew where this was going. I looked up at the title on the board. We learned about diffusion the day before. Diffusion is where molecules spread out. They go from an area of high concentration to an area of low concentration.
“Okay class. Take the bag and place it in the beaker. Then we are going to wait 15 minutes and see what happens. While we wait, you can answer the questions in your lab guide.”
- What do you think will happen with the beaker and the bag? ___________________________________________
- Why is iodine called an “indicator?” _______________________________________________________________
- What does the word “semipermeable” mean? ________________________________________________________
- Concentration is a term that refers to how much “stuff” is in a space.
With regard to starch, which is more concentrated, the bag or the beaker? ______________________
With regard to iodine, which is more concentrated, the bag or the beaker? ______________________ - What is diffusion? _____________________________________________________________________
Molecules tend to move from areas of __________ concentration to areas of __________ concentration.
15 Minutes Later - I was answering the questions in the lab guide when I noticed something about the bag. When I looked at it carefully, parts of it were turning purple. I lifted the bag out of the beaker and could see that the beaker was still the same color orange as when we started, but the white cornstarch solution was purple. It was amazing, and I knew exactly what had happened!
**Explain what happened in the experiment. Use the following words in your explanation: indicator, concentration, semipermeable, and diffusion.**

