Investigation: Taste and Signal Transduction
This page is a draft and is under active development.
( \newcommand{\kernel}{\mathrm{null}\,}\)
An Example of Signal Transduction in Humans
Chemoreception: Taste
Objectives:
- To investigate human physiological responses by observing the functions of chemoreceptors and sensory neurons associated with taste
- To investigate how taste receptors give the full taste sensation
Background
Chemoreception is the process by which organisms sense chemicals in their environment. This is often regarded as the oldest sense and is universal among animals; it is even found in bacteria and other microorganisms.
Organisms use chemoreception to accomplish a number of different tasks, including identifying suitable habitats, determining the quality of a food source, and finding a mate, Animals receive chemical information with special receptor neurons called chemoreceptors.
Chemoreception can take a number of different forms, often depending on the environment an organism lives in. In humans and most terrestrial animals, chemoreception has been refined into the senses of smell and taste. In contrast, for many organisms that have evolved living in water, there is not a big difference between the senses of smell and taste.
Gustation, the sense of taste, is closely related to the sense of smell -in fact, much of what we think of as taste is actually smell. In terrestrial vertebrates, taste is sensed through taste buds that are found in the mouth. A taste bud is actually a cluster of several cells:
- Chemoreceptor cells that occupy the center of the bud, detect the tastant (i.e., a molecule that stimulates a taste), and synapse with an afferent sensory neuron
- Support cells that form the outer wall of the taste bud, as well as some portions of the center.
In humans, approximately 10,000 taste buds are found in the epithelium of the tongue, many on the raised papillae. In contrast to smell, which is sensed by many different types of chemoreceptors, taste is sensed by only a few (4-5) – it is the combination of these receptors that leads to the variety of tastes we can sense. These taste receptors detect tastants that signal sweet, salty, sour, or bitter. Recently, a fifth taste, called umami, has been identified- the savory, meaty taste that originates from amino acids and we commonly associate with MSG (monosodium glutamate). Similar to smell, taste is sensed by the diffusion of specific molecules into the taste buds. For example “saltiness” results from the diffusion of Na+, “sourness” results from H+, and the other tastes result from a variety of organic molecules.
Contrary to a common misconception, the taste buds are not localized on the tongue into regions for the primary tastes, but rather all areas of the tongue are responsible for the primary tastes. Taste papillae can be seen on the tongue as little red dots, or raised bumps, particularly at the front of the tongue. These visible papillae on the front are actually called "fungiform" papillae because they look like little button mushrooms. There are three other kinds of papillae: foliate, circumvallate and the non-gustatory filiform papillae. A papilla is not a taste bud in itself. Rather many taste buds (which are not visible to the naked eye) are found on each papilla. At the base of each taste bud, an afferent sensory nerve invades the taste bud and branches extensively. Each afferent sensory neuron typically synapses with multiple chemoreceptor cells within the taste bud.
Receptor activation → Intracellular Signalling → Neurotransmitter Release → Gustatory Neuron
(Ca2+ entry) Activation
Signal Transduction of Taste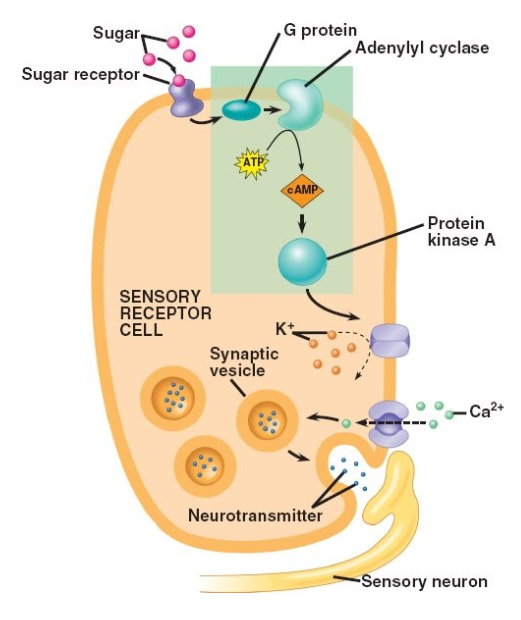
The transduction of taste signals typically involves ion channels bound in the membrane of taste chemosensory axons, including voltage-gated Na+, K+, and Ca2+ channels that produce depolarizing potentials when taste chemoreceptor cells interact with tastants.
The resulting chemoreceptor potentials raise Ca2+ to levels sufficient between the chemosensory cell and afferent sensory neuron, thus eliciting action potentials in the afferent sensory neuron (Purves, et al, 2008). In general, the higher the concentration of the tastant, the greater the depolarization of the taste cell will be.
Transduction of Sweet Taste
1. Binding of a sugar molecule to a receptor cell initiates a signal transduction pathway involving cyclic AMP and protein kinase A.
2. K+ channels in the membrane close, and the membrane depolarizes.
3. Voltage-gated calcium Ca2+ channels open, and Ca2+ diffuses into the receptor cell.
4. Synaptic vesicles release neurotransmitters, sending signals to the sensory neuron which fire the primary afferent nerve.
There are a number of different sweet receptors that respond to many organic compounds; sugars, saccharin, alcohols, some amino acids, and some lead salts such as those found in lead paint. These different receptors are what make it possible to have so many different artificial sweeteners
Procedure
To determine how taste receptors work you will drink a tea made from the Indian herb Gymnema sylvestre. This tea has a profound but reversible effect on your sense of taste. Your task is to determine what effect Gymnema has on taste perceptions and try to come up with a mechanism that explains this effect. The lab is voluntary, but we encourage your participation.
Determining How Taste Receptors Work
1. Obtain a small package of each of the following: salt, Equal®, sugar, M&Ms®, and Sweetarts®.
2. Taste these samples in the following order:
1. Salt 2. Equal® (Aspartame) 3. Sugar 4. Gummy Bears® 5. Sweetarts®
3. Rate each substance for the perception of sweet, sour, bitter, and salt on a scale from 0 to 10 in the table below. A rating of “0” represents no perceived taste whereas a rating of “10” represents a very intense taste.
4. Rinse your mouth with water between each substance in order to avoid aftertaste mixtures.
5. After your initial taste of each substance, get a sample of the Gymnema tea.
6. Swish one ounce of tea in your mouth for 30 seconds. Try to coat all areas of the mouth with the tea. Spit the tea into the sink when finished, then rinse your mouth briefly with water.
7. Beginning with salt, (and following the list above) re-taste each of the substances. Rate and record your perceptions of salty, sweet, bitter and sour for each substance on the following page.
Table 1. Rate each substance for the perception of sweet, sour, bitter, and salt on a scale from 0 to 10. A rating of “0” represents no perceived taste, a rating of “10” represents a very intense taste.
|
Tastant |
Tea |
Taste Ratings |
||||
|
Sweet |
Sour |
Salty |
Bitter |
|||
|
Salt |
Before |
|||||
|
After |
||||||
|
Aspartame |
Before |
|||||
|
After |
||||||
|
Sugar |
Before |
|||||
|
After |
||||||
|
M&Ms |
Before |
|||||
|
After |
||||||
|
Sweetarts |
Before |
|||||
|
After |
||||||
Questions
1. Based on the data you collected on yourself, what observations can you make about the effect of Gymnema sylvestre on the sense of taste? Which type(s) of taste does the tea alter?
2. For each tastant, compare your before and after tea answers.
Which (if any) tastants’ flavors were not affected by Gymnema tea?
Which (if any) tastants’ flavors were eliminated by Gymnema tea
Which (if any) tastants’ flavors were changed by Gymnema tea? In what way?
3. Read the article “Mechanism of Action of Gymnemic Acids.” Create a model (drawing) that shows how gymnema affects the taste receptors of the tongue.
4. What are possible medical uses for gymnema?
Mechanism of Action of Gymnemic Acids
 Gymnemic acid formulations have also been found useful against obesity, according to recent reports [10]. This is attributed to the ability of gymnemic acids to delay the glucose absorption in the blood. The atomic arrangement of gymnemic acid molecules is similar to that of glucose molecules. These molecules fill the receptor locations on the taste buds thereby preventing its activation by sugar molecules present in the food, thereby curbing the sugar craving. Similarly, Gymnemic acid molecules fill the receptor location in the absorptive external layers of the intestine thereby preventing the sugar molecules absorption by the intestine, which results in low blood sugar level [11].
Gymnemic acid formulations have also been found useful against obesity, according to recent reports [10]. This is attributed to the ability of gymnemic acids to delay the glucose absorption in the blood. The atomic arrangement of gymnemic acid molecules is similar to that of glucose molecules. These molecules fill the receptor locations on the taste buds thereby preventing its activation by sugar molecules present in the food, thereby curbing the sugar craving. Similarly, Gymnemic acid molecules fill the receptor location in the absorptive external layers of the intestine thereby preventing the sugar molecules absorption by the intestine, which results in low blood sugar level [11].
G. sylvestre leaves have been found to cause hypoglycemia in laboratory animals and have found a use in herbal medicine to help treat adult onset diabetes mellitus (NIDDM). When Gymnema leaf extract is administered to a diabetic patient, there is stimulation of the pancreas by virtue of which there is an increase in insulin release [12]. These compounds have also been found to increase fecal excretion of cholesterol [13], but further studies to prove clinical significance in treating hypercholesterolemia (high serum cholesterol) are required. Other uses for Gymnema leaf extract are its ability to act as a laxative, diuretic, and cough suppressant. These other actions would be considered adverse reactions when Gymnema is used for its glucose lowering effect in diabetes.
Gymnema leaf extract, notably the peptide ‘Gurmarin’, has been found to interfere with the ability of the taste buds on the tongue to taste sweet and bitter. Gymnemic acid has a similar effect. It is believed that by inhibiting the sweet taste sensation, people taking it will limit their intake of sweet foods, and this activity may be partially responsible for its hypoglycemic effect [14].
There are some possible mechanisms by which the leaves and especially Gymnemic acids from G. sylvestre exert its hypoglycemic effects are: 1) it increases secretion of insulin, 2) it promotes regeneration of islet cells, 3) it increases utilization of glucose: it is shown to increase the activities of enzymes responsible for utilization of glucose by insulin-dependant pathways, an increase in phosphorylase activity, decrease in gluconeogenic enzymes and sorbitol dehydrogenase, and 4) it causes inhibition of glucose absorption from intestine.
The gymnemic acid components are believed to block the absorption of glucose in the small intestine, the exact action being unknown. It could be involve one or more mechanisms .
The leaves are also noted for lowering serum cholesterol and triglycerides. The primary chemical constituents of Gymnema include gymnemic acid, tartaric acid, gurmarin, calcium oxalate, glucose, stigmasterol, betaine, and choline. While the water-soluble acidic fractions reportedly provide the hypoglycemic action, it is not yet clear what specific constituent in the leaves is responsible for the same. Some researchers have suggested gymnemic acid as one possible candidate, although further research is needed. Both gurmarin (another constituent of the leaves) and gymnemic acid have been shown to block sweet taste in humans. The major constituents of the plant material 3B glucuronides of different acetylated gymnemagenins, gymnemic acid a complex mixture of at least 9 closely related acidic glucosides [17–19].
Source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2170951/
References
Chaudhari,C, et al, (1996). The taste of monosodium glutamate: membrane receptors in taste buds.J.Neuroscience.16:3817-3826.
Kurihara,K and Kashiwayanagi, M. (1998). Introductory remarks on umami taste. Annals NY AcadSci.855:393-397.
Nelson, G. et al (2002). An amino-acid taste receptor. Nature 416:199-204. Purves,D, et al. (2008). Neuroscience 4 ed. Sunderland,MA:Sinauer Associates,Inc.
Smith, David and Margolskee, Robert (2001). Making sense of taste. Scientific American 284:32-39.
Reinberger, Stefanie. (2006) Bitter could be better. Scientific American 294:56-61
PBS has an apple sweetness lab based on Michael Pollan’s book Botany of Desire http://www.pbs.org/thebotanyofdesire/apple-sweetness.php
Schroeder,J and Flanery-Schroeder,E (2005) Use of the Herb Gymnema sylvestre to Illustrate the Principles of Gustatory Sensation: an Undergraduate Neuroscience Laboratory Exercise. The Journal of Undergraduate Neuroscience Education (JUNE) 3(2):A59-62
Adapted from Schroeder,J and Flanery-Schroeder,E (2005) Use of the Herb Gymnema sylvestre to Illustrate the Principles of Gustatory Sensation: an Undergraduate Neuroscience Laboratory Exercise. The Journal of Undergraduate Neuroscience Education (JUNE) 3(2):A59-62 and from a lab by David Guay.

