Case Study: Chicago Cyanide Murders
This page is a draft and is under active development.
( \newcommand{\kernel}{\mathrm{null}\,}\)
A Case Study in Cellular Respiration
Part 1: Background
In September of 1982 ,Mary Kellerman gave her 12 year old daughter a painkiller when she awoke during the night complaining of a sore throat. At 7 am the next morning, her daughter was found collapsed on the bathroom floor, and later pronounced dead.
Adam Janus, a postal worker in another Chicago suburb also died unexpectedly, though originally it was thought he had suffered from a heart attack. While his family gathered to mourn their loss, his brother and sister became ill and later died.
In the days that followed, three more unexplained deaths occurred in nearby Chicago suburbs. Investigators found that all of the victims had taken an extra strength tylenol hours before their death. They suspected that someone had tampered with the medication.

Symptoms exhibited by each of the victims included:
- weakness, dizziness, sleepiness
- flushed, bright red, skin tone
- headache
- shortness of breath and rapid breathing
- vomiting
- confusion and disorientation
Questions:
1. In your opinion, are the seven deaths connected? What additional information would you need to determine if they are connected?
2. If poison is suspected in the deaths, how would you proceed with the investigation?
Part 2: Autopsy report
The medical examiner concluded that each of the victims had died of hypoxia. Hypoxia means that the person suffered from a lack of oxygen, or they were suffocated. The reason for the hypoxia is not always clear at the first examination.
The medical examiner also showed the tissue samples from the heart, lungs, and liver showed massive cell death. On further investigation, it was shown that the tissues had major mitochondrial damage.
Even though the victims died of hypoxia, their level of oxygen in their blood was approximately 110 mm Hg. The normal range is 75100 mm Hg.
3. Recall your knowledge of the function of organelles. What function of the cells was interrupted in these patients?
4. While poison is the main suspect in the case, what are other ways a person could die of hypoxia?
5. Analyze the oxygen levels of the victims. Were the levels higher or lower than normal? How can you reconcile this observation with the cause of death being hypoxia?
Toxicology reports show that the victims had been poisoned with cyanide. The poison was traced back to extra strength tylenol where the murderer had opened the capsules and replaced acetaminophen (a pain killer) with cyanide. Cyanide acts very quickly, often killing within minutes of ingestion and authorities were slow to identify the cause of the deaths. Once the cause as identified, stores removed tylenol and other drugs from shelves. While there were many suspects, no one was ever charged with the crime and it is still an ongoing investigation. Since the Chicago Tylenol murders, drug companies have drastically changed how medicines are packaged.
Why is cyanide such an effective poison? You might be surprised to learn that it directly interferes with cellular respiration that occurs in the mitochondria.
7. Recall that the mitochondrion is sometimes called the "powerhouse" of the cell. What does this mean? Why is the mitochondrion important?
Part 3: Why Do We Need Oxygen?
It seems like a simple question, everyone knows you need to breathe to live. Have you ever thought about why oxygen is so important? The victims of the cyanide poisoning all had high levels of oxygen in their blood, but the poison was interfering with how the cells use that oxygen. To understand, we need to take a very close look at the structure of the mitochondrion.
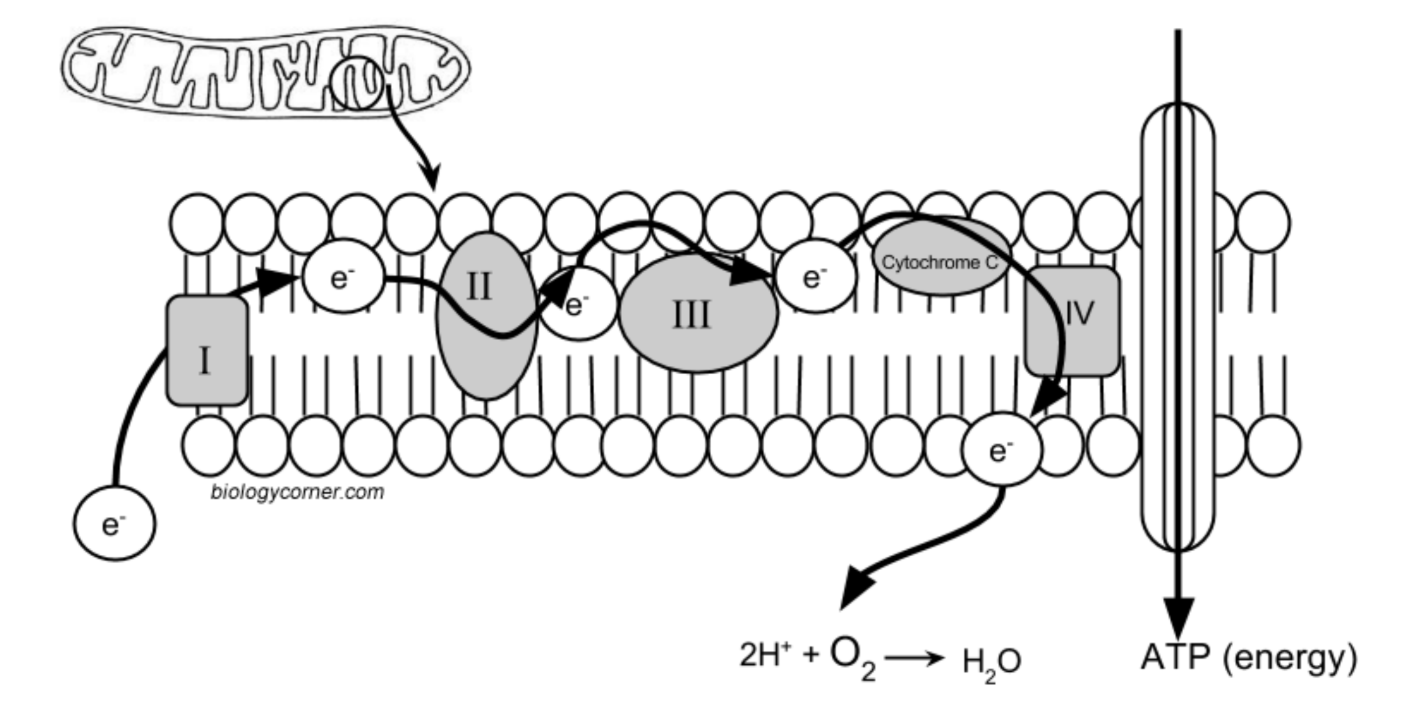
Inside the mitochondrion, there are several layers of membranes. In fact, these membranes resemble the membrane that surrounds the cell. It has a bilayer of phospholipids and embedded proteins. On the diagram above, the proteins are labeled I, II, III, IV, and Cytochrome C.
The proteins in the membrane pass electrons from one to the other, this is known as the electron transport chain. The passing of these electrons allows ATP (adenosine triphosphate) to be generated. At the end of the electron transport chain, Cytochrome C passes the electron to its final acceptor, oxygen. Oxygen then binds with proteins to create water. This process is continuous in cells, with ATP constantly being generated and oxygen being used as the final electron acceptor.
Cyanide inhibits cytochrome C, preventing the last protein from doing its job. The electron stops at the end of the chain and cannot be passed to oxygen. The whole chain grinds to a halt and no ATP can be made.
8. On the model of mitchondrion, highlight the area that is the ELECTRON TRANSPORT CHAIN. Place an X over the protein that is inhibited by cyanide. What is the relationship between the ETC and oxygen?
9. Cyanide is an extremely fast acting poison. In fact, it was developed as a suicide pill (called Lpill) during World War II so that British and American spies could avoid being captured alive. Given what you know about ATP and cellular respiration, explain why cyanide is so fast acting.
10. Given what you know about cyanide poisoning, do you think that giving a person oxygen would be an effective
treatment? Why or why not?

