15.3: Scenario for Lab 15
- Page ID
- 122720
Choose either unknown #1 or unknown #2 as your unknown for this Case Study.
Case Study
A 57-year- old female who is diabetic, a long-time smoker, and who 28 days ago had hip replacement surgery presents with swelling, pain, inflammation, and erythema at the surgical site. Examination shows she has a fever of 101°F, is exhibiting malaise, and has an increased total white blood cell count with a left shift. Ultrasonography examination indicates a deep abscess. A culture from an aspiration of the infected surgical site was taken.
Assume that unknown you are given is the culture from this patient.
MATERIALS
- 1 plate of blood agar,
- 1 novobiocin (NB) disc,
- 1 plate of mannitol salt agar,
- 1 tube of citrated rabbit plasma (coagulase test),
- materials to perform a Gram stain,
- inoculating loop
PROCEDURE (to be done in groups of 3)
[Keep in mind that in a real clinical situation other lab tests and cultures for bacteria other than those upon which this lab is based would also be done.]
TREAT EACH UNKNOWN AS A PATHOGEN!. Inform your instructor of any spills or accidents. WASH AND SANITIZE YOUR HANDS WELL before leaving the lab.
Videos reviewing techniques used in this lab:
- How to Chemically Fix a Microscope Slide with Methanol
- How to Prepare a Slide for Staining when using Bacteria from an Agar Culture
- How to Make a Gram Stain
- A Review of the Critical Decolorization Step of the Gram Stain
- Preliminary Tips for Using a Microscope
- Focusing Using Oil Immersion (1000X) Microscopy
- How to Inoculate a Blood Agar Plate and Add a NB (Novobiocin) Disc
1. Do a Gram stain on the unknown (see Lab 6). Make sure you review the instructions before you do the Gram stain. Because Enterococci and Staphylococci can sometimes look similar in Gram stains done from a plate culture, perform a catalase test on your unknown to help differentiate an Enterococcus from a Staphylococcus (see Lab 8).
- Instructions for the Gram Stain from Lab 6.
- Instructions for Focusing a Microscope from Lab 1.
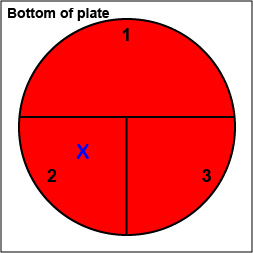
2. On the bottom of the petri plate, divide the plate into thirds with your wax marker and label as shown below. Before you streak your plate draw an "X" on the bottom of the blood agar plate in sector 2 to indicate where you will eventually place the Taxo NB disk as shown in Fig. \(\PageIndex{10}\), Step 1.
3. Using your loop, streak your unknown for isolation on a plate of Blood agar as described below.
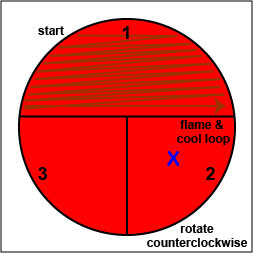
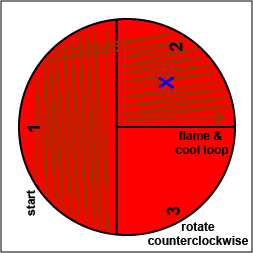
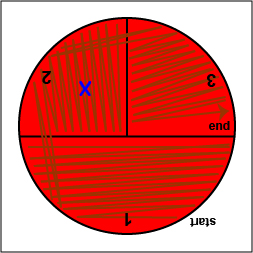
a. Using a sterile inoculating loop, streak your unknown for isolation on a blood agar plate so as to get single, isolated colonies (Fig. \(\PageIndex{10}\), step 2, Fig. \(\PageIndex{10}\), step 3, and Fig. \(\PageIndex{10}\), step 4).
| Fig. \(\PageIndex{1}\): Inoculating a Blood Agar Plate with your Unknown, Step 2 | Fig. \(\PageIndex{1}\): Inoculating a Blood Agar Plate with your Unknown, Step 3 | Fig. \(\PageIndex{1}\): Inoculating a Blood Agar Plate with your Unknown, Step 4 |
|---|---|---|
|
|
|
| Using a sterile inoculating loop, streak one-third of the blood agar plate with your unknown. Flame the loop and let it cool. | Rotate the plate counterclockwise so sector 1 is at 9:00. Using a sterile inoculating loop, spread out some of the bacteria in area 1 over area 2. Flame the loop and let it cool. | Rotate the plate counterclockwise so sector 1 is at 9:00. Using a sterile inoculating loop, spread out some of the bacteria in area 2 over area 3. |
| Copyright; Gary E. Kaiser, Ph.D. The Community College of Baltimore County, Catonsville Campus CC-BY-3.0 | ||
b. Using your inoculating loop, stab the agar 2-3 times in each of the 3 growth areas in order to detect oxygen-sensitive hemolysins (Fig. \(\PageIndex{10}\), step 5).
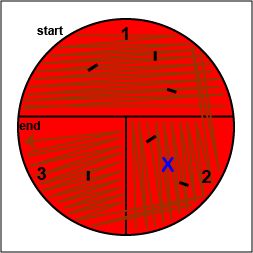
c. Place a novobiocin antibiotic disk in the area where you drew the "X" in sector 2 (Fig. 10, step 6). Tap it lightly with your loop so that the disk sticks to the agar.
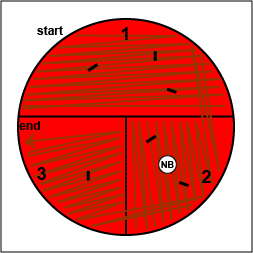
d. Incubate upside down and stacked in the petri plate holder on the shelf of the 37°C incubator corresponding to your lab section until the next lab period.
4. Streak your unknown for isolation on a plate of Mannitol Salt agar (MSA) as shown in Fig. \(\PageIndex{11}\). Incubate upside down and stacked in the petri plate holder on the shelf of the 37°C incubator corresponding to your lab section until the next lab period.
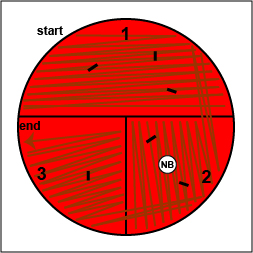
5. Inoculate a tube of citrated rabbit plasma with your unknown and incubate your test tube rack at 37°C.
Contributors and Attributions
Dr. Gary Kaiser (COMMUNITY COLLEGE OF BALTIMORE COUNTY, CATONSVILLE CAMPUS)


