12.2: Enterobacteriaceae - glucose fermenting, Gram-negative, enteric bacilli
( \newcommand{\kernel}{\mathrm{null}\,}\)
Bacteria belonging to the family Enterobacteriaceae are the most commonly encountered organisms isolated from clinical specimens. The Enterobacteriaceae is a large diverse family of bacteria belonging to the order Enterobacteriales in the class Gammaproteobacter of the phylum Proteobacter. Medically important members of this family are commonly referred to as glucose fermenting, Gram-negative, enteric bacilli, because they are Gram-negative bacilli that can ferment the sugar glucose. Many are normal flora of the intestinal tract of humans and animals while others infect the intestinal tract. Members of this family have the following characteristics in common:
1. They are Gram-negative rods. See Fig. 12.2.1.
2. If motile, they possess a peritrichous arrangement of flagella. See Fig. 12.2.2.
3. They are facultative anaerobes.
4. With few exception, they are oxidase negative.
5. All species ferment the sugar glucose but otherwise vary widely in their biochemical characteristics.
6. Most reduce nitrates to nitrites.
|
Fig 12.2.1: Escherichia coli, a Gram-negative Bacillus |
Fig. \(\PageIndex{2}\): Flagella Stain of Proteus Showing Peritrichous Arrangement of Flagella |
|---|---|
 |
 |
| Note the bacterium is surrounded by flagella (arrow). | |
| Copyright; Gary E. Kaiser, Ph.D. The Community College of Baltimore County, Catonsville Campus CC-BY-3.0 | |
The Enterobacteriaceae represent a very large and diverse family of bacteria. Some of the more common clinically important genera of the family Enterobacteriaceae include:
| Salmonella | Citrobacter | Morganella |
| Shigella | Enterobacter | Yersinia |
| Proteus | Serratia | Edwardsiella |
| Escherichia | Klebsiella | Providencia |
Several genera of Enterobacteriaceae are associated with gastroenteritis and food-borne disease. These include:
- Salmonella.
- Shigella.
- Certain strains of Escherichia coli.
- Certain species of Yersinia.
All intestinal tract infections are transmitted by the fecal-oral route.
There are two species of Salmonella, Salmonella enterica and Salmonella bongori. Any infection caused by Salmonella is called a salmonellosis. Non-typhoidal Salmonella accounts for an estimated 520 cases per 100,000 population. CDC estimates that there are approximately 1,200,000 cases of non-typhoidal salmonellosis each year in the U.S resulting in 450 deaths. Since many different animals carry Salmonella in their intestinal tract, people usually become infected from ingesting improperly refrigerated, uncooked or undercooked poultry, eggs, meat, dairy products, vegetables, or fruit contaminated with animal feces.
Enteritis is the most common form of salmonellosis. Symptoms generally appear 6-48 hours after ingestion of the bacteria and include vomiting, nausea, non-bloody diarrhea, fever, abdominal cramps, myalgias, and headache. Symptoms generally last from 2 days to 1 week followed by spontaneous recovery. All species of Salmonella can cause bacteremia but S. enterica serotype Typhi, isolated only from humans, frequently disseminates into the blood causing a severe form of salmonellosis called typhoid fever. About 400 cases of typhoid fever occur each year in the U.S. but approximately 75% of these are acquired while traveling internationally.
Salmonella serotyping is a subtyping method of identification based on the identification of distinct cell wall, flagellar, and capsular antigens with known antiserum, as will be discussed in Lab 17. Salmonella serotypes Enteritidis and Typhimurium are the two most common serotypes in the United States, accounting for approximately 35 to 40% of all infections confirmed by laboratory culture. As mentioned above, S. enterica serotype Typhi is responsible for typhoid fever.
Any Shigella infection is called a shigellosis. Unlike Salmonella, which can infect many different animals, Shigella only infects humans and other higher primates. There are approximately 14,000 laboratory cases of shigellosis a year reported in the US with an estimated 450,000 total cases and 70 deaths.
Shigellosis frequently starts with a watery diarrhea, fever, and abdominal cramps but may progress to dysentery with scant stools containing blood, pus, and mucus. The incubation period is 1-3 days. Initial profuse watery diarrhea typically appears first as a result of enterotoxin. Within 1-2 days this progresses to abdominal cramps, with or without bloody stool. Classic shigellosis presents itself as lower abdominal cramps and stool abundant with blood and pus develops as the Shigella invade the mucosa of the colon.
Escherichia coli is one of the dominant normal flora in the intestinal tract of humans and animals. Some strains, however, can cause infections of the intestines while others are capable of causing infections outside the intestines. Extraintestinal pathogenic E. coli cause such opportunistic infections as urinary tract infections, wound infections, and septicemia and will be discussed in greater detail below. Intestinal or diarrheagenic E. coli cause infections of the intestinal tract. Diarrheagenic E. coli include:
- Enterotoxigenc E. coli (ETEC) produce enterotoxins that cause the loss of sodium ions and water from the small intestines resulting in a watery diarrhea. It is an important cause of diarrhea in impoverished countries. ETEC accounts for between 11-15% of cases of traveler's diarrhea in persons visiting developing countries and 30-45% of cases among those visiting Mexico. There are approximately 80,000 cases of ETEC a year in the U.S.
- Enteropathogenic E. coli (EPEC) causes an endemic diarrhea in in impoverished countries, especially in infants younger than 6 months of age. The bacterium disrupts the normal microvilli on the epithelial cells of the small intestines resulting in maladsorbtion and diarrhea. They do not produce enterotoxin or shiga toxin and are not invasive. It is rare in industrialized countries.
- Enteroaggregative E. coli (EAEC) is a cause of endemic diarrhea in children in impoverished countries and industrialized countries. EAEC causes around 30% of cases of traveler's diarrhea. It is also responsible for a persistent diarrhea in people infected with HIV. It probably causes diarrhea by adhering to mucosal epithelial cells of the small intestines and interfering with their function.
- Enteroinvasive E. coli (EIEC) invade and kill epithelial cells of the colon usually causing a watery diarrhea but sometimes progressing to a dysentery-type syndrome with blood in the stool. It occurs mostly in impoverished countries and is rare in industrialized countries.
- Shiga toxin-producing E. coli (STEC) such as E. coli 0157:H7, produce a shiga toxin that kills epithelial cells of the colon causing hemorrhagic colitis, a bloody diarrhea. In rare cases, the shiga toxin enters the blood and is carried to the kidneys where, usually in children, it damages vascular cells and causes hemolytic uremic syndrome. E. coli 0157:H7 is thought to cause more than 20,000 infections and up to 250 deaths per year in the U.S.
Several species of Yersinia, such as Y. enterocolitica and Y. pseudotuberculosis are also causes of diarrheal disease.
Many other genera of the family Enterobacteriaceae are normal microbiota of the intestinal tract and are considered opportunistic pathogens. The most common genera of Enterobacteriaceae causing opportunistic infections in humans include the following:
- Escherichia coli.
- Proteus.
- Enterobacter.
- Klebsiella.
- Citrobacter.
- Serratia.
They act as opportunistic pathogens when they are introduced into body locations where they are not normally found, especially if the host is debilitated or immunosuppressed. They all cause the same types of opportunistic infections, namely:
- Urinary tract infections.
- Wound infections.
- Pneumonia.
- Septicemia.
These normal flora Gram-negative bacilli, along with Gram-positive bacteria such as Enterococcus species (see Lab 14) and Staphylococcus species (see Lab 15), are among the most common causes of health-care-associated infections (formerly called nosocomial infections).
According to the Centers for Disease Control and Prevention (CDC) Health-care-associated infection's website, "In American hospitals alone, health-care-associated infections account for an estimated 1.7 million infections and 99,000 associated deaths each year. Of these infections:
- 32 percent of all health-care-associated infection are urinary tract infections (UTIs.)
- 22 percent are surgical site infections.
- 15 percent are pneumonia (lung infections.)
- 14 percent are bloodstream infections.
Diseases and Organisms in Healthcare Settings; from CDC
Most patients who have healthcare-associated infections are predisposed to infection because of invasive supportive measures such as urinary catheters, intravascular lines, and endotracheal intubation.
By far, the most common Gram-negative bacterium causing nosocomial infections is Escherichia coli. E. coli causes between 70 and 90% of both upper and lower urinary tract infections (UTIs). It is also a frequent cause of abdominal wound infections and septicemia. Depending on the facility, E. coli is responsible for between 12% and 50% of all healthcare-associated infections.
However, according to a 2008 study, Enterobacteriaceae other than E. coli were responsible for 7 of the 10 most common Gram-negative organisms isolated from urinary tract, respiratory tract, and bloodstream infections from intensive care unit patients between 2002 and 2008 in the United States. These include Klebsiella pneumoniae (15%), Enterobacter cloacae (9%), Serratia marcescens (6%), Enterobacter aerogenes (4%), Proteus mirabilis (4%), Klebsiella oxytoca (3%), and Citrobacter freundii (2%). Furthermore, the National Healthcare Safety Network reported K. pneumoniae (6%) , Enterobacter spp. (5%), and K. oxytoca (2%) among the top 10 most frequently isolated health care-associated infections between the years between 2006 and 2007.
1. Urinary Tract Infections
The most common infection caused by opportunistic Enterobacteriaceae is a urinary tract infection (UTI). UTIs account for more than 8, 000,000 physician office visits per year in the U.S and as many as 100,000 hospitalizations. Among the non-hospitalized and non-debilitated population, UTIs are more common in females because of their shorter urethra and the closer proximity between their anus and the urethral opening. (Over 20 percent of women have recurrent UTIs.) However, anyone can become susceptible to urinary infections in the presence of predisposing factors that cause functional and structural abnormalities of the urinary tract. These abnormalities increase the volume of residual urine and interfere with the normal clearance of bacteria by urination. Such factors include prostate enlargement, sagging uterus, expansion of the uterus during pregnancy, paraplegia, spina bifida, scar tissue formation, and catheterization. Between 35 and 40 percent of all nosocomial infections, about 900,000 per year in the U.S., are UTIs and are usually associated with catheterization.
E. coli and Staphylococcus saprophyticus (a Gram-positive staphylococcus that will be discussed in Lab 15) cause around 90 percent of all uncomplicated UTIs. Most of the remaining uncomplicated UTIs are caused by other Gram-negative enterics such as Proteus mirabilis and Klebsiella pneumoniae or by Enterococcus faecalis (a Gram-positive streptococcus that will be discussed in Lab 14). E. coli is responsible for more than 50 percent of healthcare-associated UTIs. Other causes of hospital-acquired UTIs include other species of Enterobacteriaceae (such as Proteus, Enterobacter, and Klebsiella), Pseudomonas aeruginosa (discussed below), Enterococcus species (discussed in lab 14), Staphylococcus saprophyticus (discussed in Lab 15), and the yeast Candida (discussed in lab 9).
The traditional laboratory culture standard for a UTI has been the presence of more than 100,000 CFUs (colony-forming units; see Lab 4) per milliliter (ml) of midstream urine, or any CFUs from a catheter-obtained urine sample. More recently, this has been modified and counts of as few as 1000 colonies of a single type per ml or as little as 100 coliforms per ml are now considered as indicating a UTI.
2. Wound Infections
Wound infections are due to fecal contamination of external wounds or a result of wounds that cause trauma to the intestinal tract, such as surgical wounds, gunshot wounds, and knife wounds. In the latter case, fecal bacteria get out of the intestinal tract and into the peritoneal cavity causing peritonitis and formation of abscesses on the organs found in the peritoneal cavity.
3. Pneumonia
Although they sometimes cause pneumonia, the Enterobacteriaceae account for less than 5% of the bacterial pneumonias requiring hospitalization.
4. Bloodstream Infections
Gram-negative septicemia is a result of these opportunistic Gram-negative bacteria getting into the blood. They are usually introduced into the blood from some other infection site, such as an infected kidney, wound, or lung. Looking at patients that develop septic shock:
- Lower respiratory tract infections are the source in about 25% of patients.
- Urinary tract infections are the source in about 25% of patients.
- Soft tissue infections are the source in about 15% of patients.
- Gastrointestinal infections are the source in about 15% of patients.
- Reproductive tract infections are the source in about 10% of patients.
- Foreign bodies (intravascular lines, implanted surgical devices, etc.) are the source in about 5% of patients.
There are approximately 750,000 cases of septicemia per year in the U.S. and 200,000 cases of septic shock. Septic shock results in approximately 100,000 deaths per year in the U.S. Approximately 45 percent of the cases of septicemia are due to Gram-negative bacteria. Klebsiella, Proteus, Enterobacter, Serratia, and E. coli, are all common Enterobacteriaceae causing septicemia. (Another 45 percent are a result of Gram-positive bacteria (see Labs 14 and 15) and 10 percent are due to fungi, mainly the yeast Candida (see Lab 9).
In the outer membrane of the Gram-negative cell wall, the lipid A moiety of the lipopolysaccharide (LPS) functions as an endotoxin (see Fig 12.2.3). Endotoxin indirectly harms the body when massive amounts are released during severe Gram-negative infections. This, in turn, causes an excessive cytokine response.
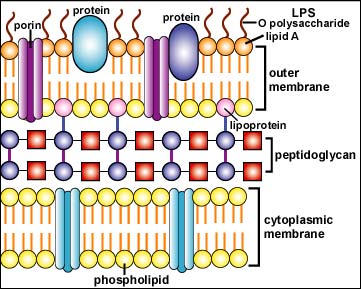
1. The LPS released from the outer membrane of the Gram-negative cell wall first binds to a LPS-binding protein circulating in the blood and this complex, in turn, binds to a receptor molecule (CD14) found on the surface of body defense cells called macrophages (see Fig. 12.2.4) located in most tissues and organs of the body.
2. This is thought to promote the ability of the toll-like receptor TLR-4 to respond to the LPS, triggering the macrophages to release various defense regulatory chemicals called cytokines, including tumor necrosis factor-alpha (TNF-alpha), interleukin-1 (IL-1), interleukin-6 (IL-6), and interleukin-8 (IL-8), and platelet-activating factor (PAF). The cytokines then bind to cytokine receptors on target cells and initiate inflammation and activate both the complement pathways and the coagulation pathway (see Fig. 12.2.4).
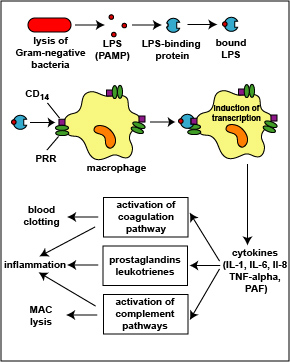

3. The complex of LPS and LPS binding protein can also attach to molecules called CD14 on the surfaces of phagocytic white blood cells called neutrophils causing them to release proteases and toxic oxygen radicals for extracellular killing. Chemokines such as interleukin-8 (IL-8) also stimulate extracellular killing. In addition, LPS and cytokines stimulate the synthesis of a vasodilator called nitric oxide.

During minor local infections with few bacteria present, low levels of LPS are released leading to moderate cytokine production by the monocytes and macrophages and in general, promoting body defense by stimulating inflammation and moderate fever, breaking down energy reserves to supply energy for defense, activating the complement pathway and the coagulation pathway, and generally stimulating immune responses (see Fig. 12.2.4 above). Also as a result of these cytokines, circulating phagocytic white blood cells such as neutrophils and monocytes stick to the walls of capillaries, squeeze out and enter the tissue, a process termed diapedesis. The phagocytic white blood cells such as neutrophils then kill the invading microbes with their proteases and toxic oxygen radicals.
However, during severe systemic infections with large numbers of bacteria present, high levels of LPS are released resulting in excessive cytokine production by the monocytes and macrophages and this can harm the body (see Fig. 12.2.5). In addition, neutrophils start releasing their proteases and toxic oxygen radicals that kill not only the bacteria, but the surrounding tissue as well. Harmful effects include high fever, hypotension, tissue destruction, wasting, acute respiratory distress syndrome (ARDS), disseminated intravascular coagulation (DIC), and damage to the vascular endothelium resulting in shock, multiple system organ failure (MOSF), and often death.

Figure 12.2.5: Harmful Effects of Lipopolysaccharide (LPS; Endotoxin) Released from the Gram-Negative Cell Wall. The lysis of Gram-negative bacteria causes them to release lipopolysaccharide (LPS; endotoxin) from the outer membrane of their cell wall. The LPS binds to a LPS-binding protein circulating in the blood and this complex, in turn, binds to a receptor molecule (CD14) found on the surface of body defense cells called macrophages. This triggers the macrophages to release various defense regulatory chemicals called cytokines, including IL-1, IL-6, IL-8, TNF-alpha, and PAF. The cytokines then bind to cytokine receptors on target cells stimulating the production of inflammatory mediators such as prostaglandins and leukotrienes as well as activating both the complement pathways and the coagulation pathway. Excessive production of clotting factors may lead to ARDS and DIC while an overproduction of prostaglandins, leukotrienes, and complement proteins can damage the vascular endothelium resulting in shock and MOSF. (LPS, lipopolysaccharide; IL-1, interleukin-1; IL-6, interleukin-6; IL-8, interleukin-8, TNF-alpha, tumor necrosis factor-alpha; PAF, platelet-activating factor; ARDS, acute respiratory distress syndrome; DIC, disseminated intravascular coagulation; MOSF, multiple organ system failure.) ( Copyright; Gary E. Kaiser, Ph.D. The Community College of Baltimore County, Catonsville Campus CC-BY-3.0 )
This excessive inflammatory response is referred to as Systemic Inflammatory Response Syndrome or SIRS. Death is a result of what is called the shock cascade. The sequence of events is as follows:
The release of excessive levels of inflammatory cytokines in response to PAMPs binding to PRRs during a systemic infection results in:
1. A drop in blood volume or hypovolemia. This is caused by the following events:
a. Extracellular killing by neutrophils damages the capillary walls resulting in blood and plasma leaving the bloodstream and entering the surrounding tissue.
b. Depletion of clotting factors during disseminated intravascular coagulation (DIC) can lead to hemorrhaging as the capillaries are damaged.
c. Prolonged vasodilation results in plasma leaving the bloodstream and entering the surrounding tissue.
2. A drop in blood pressure or hypotension. This is a result of the following events:
a. Prolonged vasodilation causes decreased vascular resistance within blood vessels decreases blood pressure.
b. High levels of TNF, inhibit vascular smooth muscle tone and myocardial contractility decreasing the ability of the heart to pump blood throughout the body.
c. Hypovolemia from capillary damage, plasma leakage, and hemorrhaging.
3. The inability to deliver nutrients and oxygen to body cells or hypoperfusion. This is a result of the following events:
a. Activation of the blood coagulation pathway can cause clots called microthrombi to form within the blood vessels throughout the body causing disseminated intravascular coagulation (DIC) which blocks the flow of blood through the capillaries and, as mentioned above, depletion of clotting factors can lead to hemorrhaging in many parts of the body.
b. Increased capillary permeability as a result of vasodilation in the lungs, as well as neutrophil-induced injury to capillaries in the alveoli leads to acute inflammation, pulmonary edema, and loss of gas exchange in the lungs (acute respiratory distress syndrome or ARDS). As a result, the blood does not become oxygenated.
c. Hypovolemia decreases the volume of circulating blood and leads to hypotension.
d. Hypotension decreases the pressure needed to deliver blood throughout the body.
6. Hypoperfusion in the liver can result in a drop in blood glucose level from liver dysfunction. Glucose is needed for ATP production during glycolysis and aerobic respiration. A drop in glucose levels can result in decreased ATP production and insufficient energy for cellular metabolism.
7. The lack of oxygen delivery as a result of hypoperfusion causes cells to switch to fermentation for energy production. The lactic acid end products of fermentation may lead to lactic acidosis and a blood pH to low for the functioning of the enzymes involved in cellular metabolism. This can result in irreversible cell death.
Collectively, this can result in :
- end-organ ischemia: a restriction in blood supply that results in damage or dysfunction of tissues or organs,
- multiple system organ failure (MSOF),
- death.
Both pili and surface proteins in the Gram-negative cell wall function as adhesins, allowing the bacterium to adhere intimately to host cells and other surfaces in order to colonize and resist flushing. Some Gram-negative bacteria also produce invasins, allowing some bacteria to invade host cells. Motility, capsules, biofilm formation, and exotoxins also play a role in the virulence of some Enterobacteriaceae.
Many of the Enterobacteriaceae also possess R (resistance) plasmids. These plasmids are small pieces of circular non-chromosomal DNA that may code for multiple antibiotic resistance In addition, the plasmid may code for a sex pilus, enabling the bacterium to pass R plasmids to other bacteria by conjugation. Between 50 and 60 percent of the bacteria causing healthcare-associated infections are antibiotic resistant.


Figure 12.2.6B: Animation illustrating extracellular killing by neutrophils triggered by the binding of LPS and chemokines to receptors on neutrophils. ( Copyright; Gary E. Kaiser, Ph.D. The Community College of Baltimore County, Catonsville Campus CC-BY-3.0 )
The identification of lactose-fermenting Gram-negative rods belonging to the bacterial family Enterobacteriaceae (bacteria commonly referred to as coliforms) in water is often used to determine if water has been fecally contaminated and, therefore, may contain disease-causing pathogens transmitted by the fecal-oral route. The procedure for this is given in Appendix E.
Contributors and Attributions
Dr. Gary Kaiser (COMMUNITY COLLEGE OF BALTIMORE COUNTY, CATONSVILLE CAMPUS)

