9.6: Procedure
- Page ID
- 123391
Today we will use three agars to grow our yeast: Saboraud Dextrose agar (SDA), Mycosel agar, and Rice Extract agar. Saboraud Dextrose agar (SDA) is an agar like trypticase soy agar but with a higher sugar concentration and a lower pH, both of which inhibit bacterial growth but promote fungal growth. SDA, therefore, is said to be selective for fungi.
Another medium, Mycosel agar, contains chloramphenicol to inhibit bacteria and cycloheximide to inhibit most saprophytic fungi. Mycosel agar, therefore, is said to be selective for pathogenic fungi.
Rice Extract agar with polysorbate 80 stimulates the formation of hyphae, blastoconidia, and chlamydoconidia (see Fig. \(\PageIndex{1}\)), structures unique to C. albicans, and may be used in its identification. The speciation of Candida is based on sugar fermentation patterns.
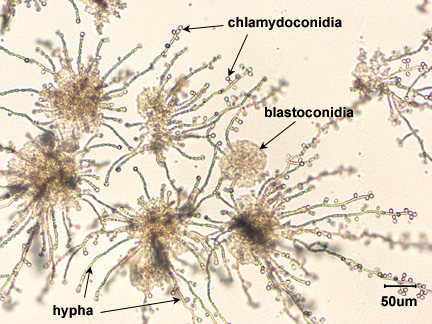
Coverslips, alcohol, forceps, and one plate each of Saboraud Dextrose agar, Mycosel agar, and Rice Extract agar.
Trypticase Soy broth cultures of Candida albicans and Saccharomyces cerevisiae.
PROCEDURE (to be done in pairs)
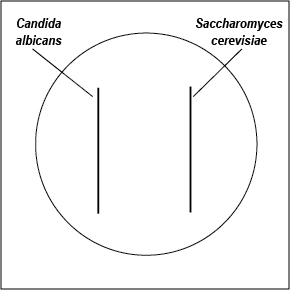
- With a wax marker, divide a Saboraud Dextrose agar and a Mycosel agar plate in half. Using a sterile swab, inoculate one half of each plate with C. albicans and the other half with S. cerevisiae as shown in Fig. \(\PageIndex{2}\). Incubate the two plates upside down and stacked in the petri plate holder on the shelf of the 37°C incubator corresponding to your lab section until the next lab period.
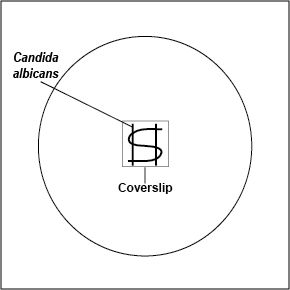
- Using your inoculating loop, streak two parallel lines of Candida albicans approximately 1.5 cm long and 1.0 cm apart onto the surface of a plate of Rice Extract agar. Sterilize the inoculating loop and let it cool. Using the sterile loop, make an S-shaped streak lightly back and forth across the two parallel streak lines as shown in Fig. \(\PageIndex{3}\). Pick up a glass coverslip with forceps, dip the coverslip in alcohol, and ignite with the flame of your butane lighter. Let the coverslip cool for a few seconds and place it over a portion of the streak line so that the plate can be observed directly under the microscope after incubation. Incubate upside down at room temperature for 3-5 days and examine microscopically.
|
Fig \(\PageIndex{1}\): Inoculating SDA and Mycosel Agar Plates with Candida albicans and Saccharomyces cerevisiae |
Fig. \(\PageIndex{2}\): Inoculating a Rice Extract Agar Plate with Candida albicans |
 |
 |
| Streak with sterile swabs and incubate upside down and stacked in the petri plate holder on the shelf of the 37°C incubator corresponding to your lab section. | Using your inoculating loop, streak two parallel lines approximately 1.5 cm long and 1.0 cm apart onto the surface of a plate of Rice Extract agar. Make an S-shaped streak lightly back and forth across the two parallel streak lines. Pick up a glass coverslip with forceps, dip the coverslip in alcohol, and ignite with the flame of your butane lighter. Let the coverslip cool for a few seconds and place it over a portion of the streak line so that the plate can be observed directly under the microscope after incubation. Incubate upside down at room temperature. |
| Copyright; Gary E. Kaiser, Ph.D. The Community College of Baltimore County, Catonsville Campus CC-BY-3.0 | |
3. Observe the following demonstrations:
|
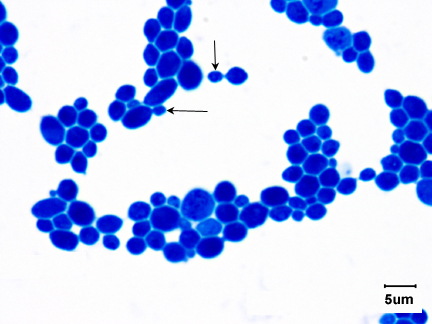
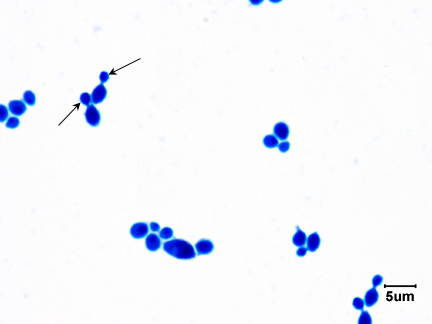
Fig \(\PageIndex{3}\): Saccharomyces cerevisiae |
Fig. \(\PageIndex{4}\): Candida albicans |
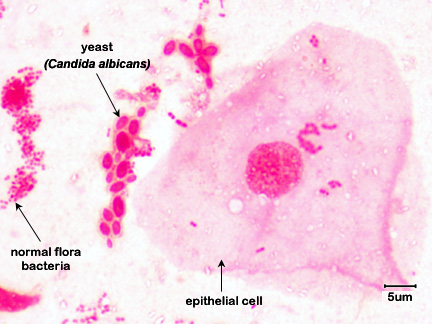
Fig \(\PageIndex{5}\):Mouth Smear of a Person with Thrush |
Fig \(\PageIndex{6}\): Lung of a Mouse Infected with Candida |
 |
 |
 |
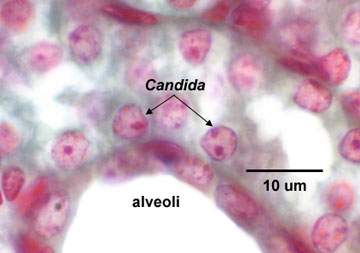 |
| Note budding yeast (arrows) | Note budding yeast (arrows). | Note budding yeast (arrows). | Note yeast (arrows). |
| Copyright; Gary E. Kaiser, Ph.D. The Community College of Baltimore County, Catonsville Campus CC-BY-3.0 | |||
|
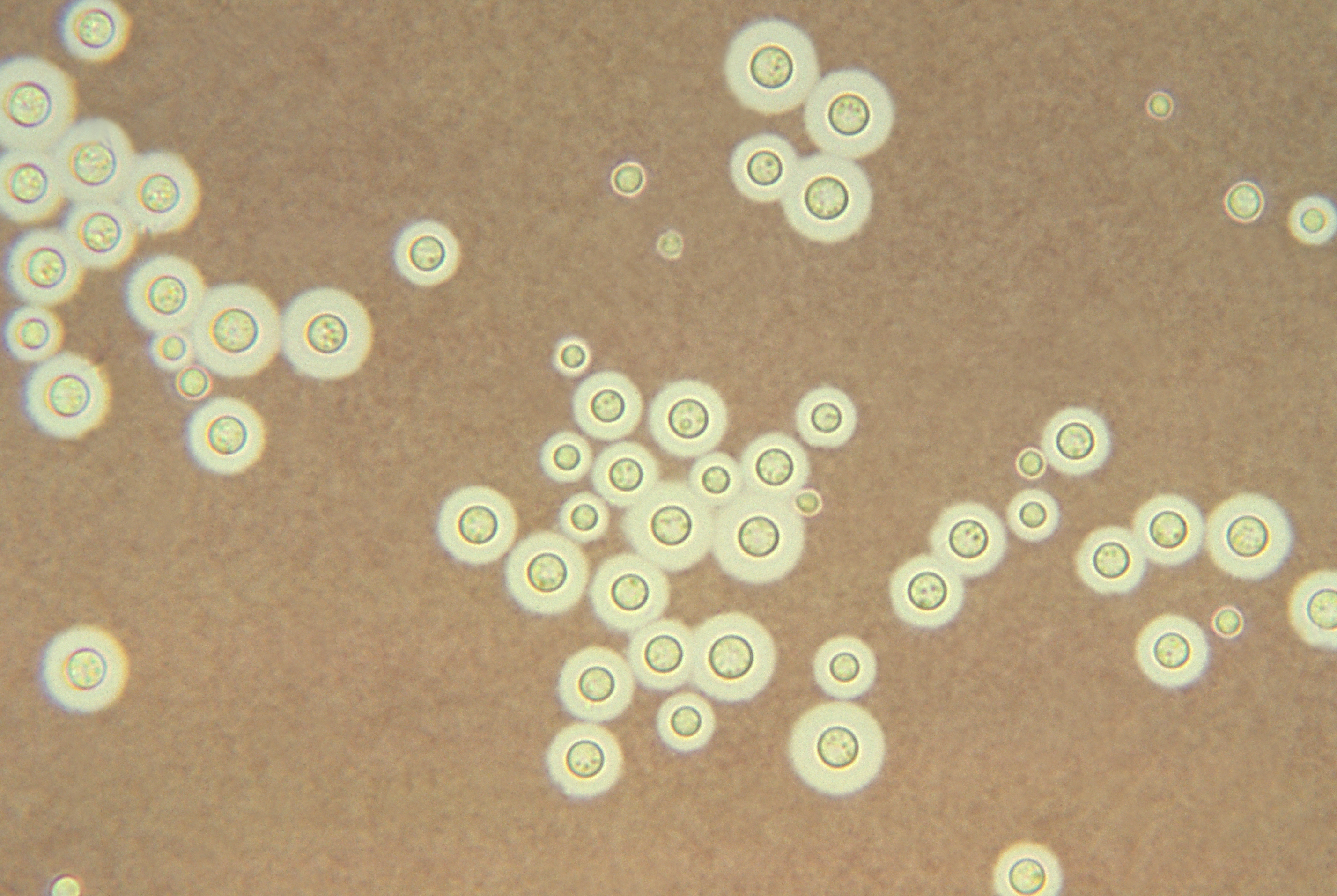
Fig \(\PageIndex{7}\): India ink stain of encapsulated Cryptococcus neoformans |
Fig. \(\PageIndex{8}\): Candida albicans |
 |
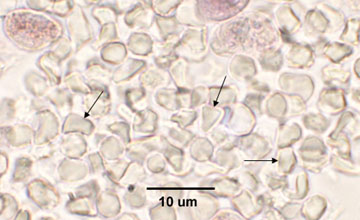 |
| Note encapsulated yeast. | Cysts of Pneumocystis jiroveci in lung tissue, Gomori methenamine silver stain method. The walls of the cysts are stained black and often appear crescent shaped or like crushed ping-pong balls. The intracystic bodies are not visible with this stain. |
| By Content Providers: CDC/Dr. Leanor Haley [Public domain]. Courtesy of the Centers for Disease Control and Prevention. |
Copyright; Gary E. Kaiser, Ph.D. The Community College of Baltimore County, Catonsville Campus CC-BY-3.0 |
Contributors and Attributions
Dr. Gary Kaiser (COMMUNITY COLLEGE OF BALTIMORE COUNTY, CATONSVILLE CAMPUS)

