1.3: Biological Molecules
( \newcommand{\kernel}{\mathrm{null}\,}\)
Introduction:
(Modified from http://www.biology101.org/biologystu...ocksoflife.php)
Biological systems are made up of four major classes of macromolecules: carbohydrates, lipids, proteins, and nucleic acids (nucleic acids will be covered separately later).
Carbohydrates: 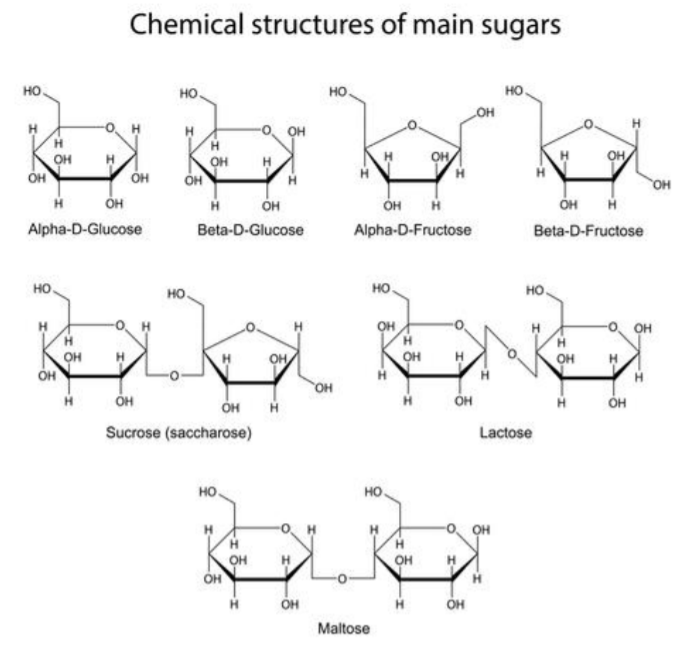
Carbohydrates are the most abundant macromolecules on earth, and the source of immediate energy needs in living systems. Carbohydrates also participate in defining the structure of cells and living systems. There are 3 general chemical grouping for carbohydrates: monosaccharides, disaccharides, and polysaccharides.
Monosaccharides, also referred to as simple sugars, are made up of a single sugar molecule. The major example of a monosaccharide is glucose (C6H12O6). Other monosaccharides include isomers of glucose, such as fructose and galactose. Monosaccharides are transported in the blood of animals, broken down to produce chemical energy inside the cell, and can also be found within other macromolecules, such as nucleic acids.
Disaccharides are composed of two single monomers of sugar linked together. Examples of disaccharides are maltose (glucose + glucose) and sucrose (glucose + fructose). Disaccharides are broken down into their subunits for use inside living systems.
Polysaccharides are polymers, or long chains of sugar monomers linked together, and are stored inside the cell for future energy use. In plants, the major storage polysaccharide is starch, while in animals it is glycogen. Plants also contain cellulose, which is the most abundant of all carbohydrates. Cellulose is found in the plant cell wall, where it provides structure and support to the plant cell.
Lipids:
Lipids are nonpolar macromolecules; thus they are insoluble in water. They include oils and fats, phospholipids, and steroids. Fats and oils are triglycerides, composing of one glycerol and 3 fatty acids. A fatty acid is a long chain of carbon-hydrogen (C-H) bonds, with a carboxyl group (-COOH) at one end. Fatty acids can be classified as saturated or unsaturated. Fatty acids are saturated when they do not contain any double bonds between the carbons and unsaturated when they contain double bonds. An example of a saturated fat is butter, while an example of an unsaturated fat is vegetable oil.
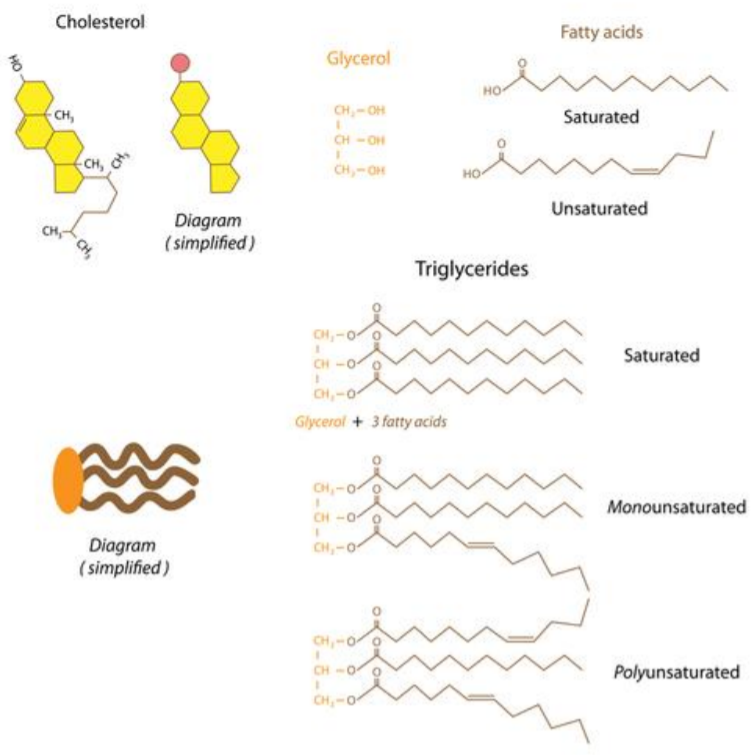
Phospholipids are found primarily in the cell membranes of living systems, of which they are the major component. Structurally, a phospholipid contains a hydrophilic head and a hydrophobic tail. The head of the phospholipid contains a phosphate group while the tail is typically a diglyceride. The cell membrane is a double layer of phospholipids in which the tails are turned inwards and the heads are exposed to the intracellular and extracellular environments.
Steroids are lipids which are typically made up of fused hydrocarbon rings. Each type of steroid is different in the type of chemically active functional groups that it contains. Examples of steroids include cholesterol, estrogen, and testosterone. Cholesterol found in the cell membrane of animals, where it provides structural support. Cholesterol is also the precursor for other steroids, such as testosterone and estrogen. Some vitamins, such as Vitamin D, are also classified as steroids.
Proteins:
Proteins are polymers of amino acids. Amino acids are small molecules that contain an amine (-NH2), a carboxyl acid (-COOH), and a side chain (R). There are twenty naturally occurring amino acids, and each amino acid has a unique side-chain or R-group. Amino acids are connected by peptide bonds to form protein polymers. This gives rise to different levels of structure for proteins. The primary (1°) structure of a protein is a made up of a string of amino acids connected via peptide bonds. The secondary (2°) structure of a protein is formed by the coiling and folding of the 1° structure, due to hydrogen bonding. At this level, structures called alpha-helices and beta-sheets are visible. The tertiary (3°) structure of a protein is formed by interactions between the components of the 2° structure. Some proteins have quaternary (4°) structure, which includes the assembly of multiple individual subunits to form the functional protein.
Proteins have numerous biological functions. The common types of proteins that are found in biological systems are enzymes, antibodies, transport proteins, regulatory proteins, and structural proteins.
Part 1: Testing For Simple Sugars
- Benedict’s solution turns orange-brown in the presence of simple sugars.
Test samples: distilled water, glucose, starch, orange juice, regular soda, diet soda, unknown
Note
Shake the starch before using it.
1. Fill a beaker about halfway with water and bring it to a gentle boil on the hot plate.
2. Label tubes. Use a wax pencil to label the tubes with the test and sample ID; label at the volume markings listed below.
3. Add 2 mL. sample to the tube (2 cm).
4. Add 2 mL. (2 cm.) Benedict’s solution and swirl to mix. Record the color in the table below.
5. Put the tube in the water and heat for 3 minutes.
6. Use test tube clamps to remove tubes from heat and record color.
Note
Dispose of waste in waste beaker.
| Tube | Sample | Initial Color | Color After Heating | Conclusion |
| 1 | Water | |||
| 2 | Glucose | |||
| 3 | Starch | |||
| 4 | Orange Juice | |||
| 5 | Regular Soda | |||
| 6 | Diet Soda | |||
| 7 | Unknown |
Part 2: Testing For Starch
- Lugol’s reagent (IKI solution) turns dark blue/black in the presence of starch.
Test samples: distilled water, glucose, starch, orange juice, regular soda, diet soda, unknown
Note
Shake starch before using.
1. Label tubes Use a wax pencil to label the tubes with the test and sample ID; label at the volume markings listed below.
2. Add 3 mL. samples (3 cm.) and record color in the chart.
3. Add 9 drops Lugol’s reagent and swirl to mix.
4. Record color in the table below.
| Tube | Sample | Initial Color | Color After IKI | Conclusion |
| 1 | Water | |||
| 2 | Glucose | |||
| 3 | Starch | |||
| 4 | Orange Juice | |||
| 5 | Regular Soda | |||
| 6 | Diet Soda | |||
| 7 | Unknown |
Part 3: Testing For Lipids
A. Paper Test
- Paper turns translucent (gets a grease spot) in presence of lipids.
Test samples: distilled water, vegetable oil, cream, and unknown.
1. Put a drop of each substance on a brown paper towel. Rub in the cream.
2. Put the towel off to the side and let it sit for 10 minutes.
3. Record the appearance of the bag where you placed the spots in the table below.
| Sample | Appearance After Drying | Conclusion |
| Water | ||
| Oil | ||
| Cream | ||
| Unknown |
B. Sudan IV Test
- Two layers form in the presence of lipids. Red dye concentrates in the top layer.
Test samples: distilled water, vegetable oil, cream, and unknown.
1. Label tubes Use a wax pencil to label the tubes with the test and sample ID; label at the volume markings listed below.
2. Add 3 mL. (3 cm.) distilled water to each tube.
3. Add 3 mL. (3 cm.) of sample to the tube (total of 6 ml per tube).
4. Add 9 drops of Sudan IV and shake (must mix well).
5. Add 2 mL. (2 cm.) water.
6. Record results in the table below.
| Tube | Sample | Appearance After Sudan IV | Conclusion |
| 1 | Water | ||
| 2 | Oil | ||
| 3 | Cream | ||
| 4 | Unknown |
Part 4: Testing For Proteins
- Biuret’s reagent turns dark blue-purple in the presence of peptide bonds.
Test samples: distilled water, starch, egg albumin, glucose, regular soft drink, unknown
Note
Shake starch before using.
1. Label tubes Use a wax pencil to label the tubes with the test and sample ID; label at the volume markings listed below.
2. Add 1 mL. (1 cm.) sample to the tube.
3. Add 10 drops Biuret’s and swirl to mix.
4. Wait 2 minutes, then record color.
5. Dispose of Biuret waste in the container.
| Tube | Sample | Initial Color | Color After Biuret | Conclusion |
| 1 | Water | |||
| 2 | Starch | |||
| 3 | Egg Albumin | |||
| 4 | Glucose | |||
| 5 | Regular Soda | |||
| 6 | Unknown |
Unknown:
Look back over the results for the unknown sample from each of the previous tests. Based on the data, what combination of macromolecules is present in the unknown mixture? ____________________________________________
Questions:
1. The monomers that make up carbohydrates are:
2. The monomers that make up proteins are:
3. Which test could you use to distinguish between diet and regular soda? What would the test detect?
4. What is the Biuret test actually detecting? Be as specific as possible.
5. Each test included a sample that was just water. Why is it important to include a water-only sample for each test?


