6.3: The Rock Cycle
- Page ID
- 69396
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The rock components of the crust are slowly but constantly being changed from one form to another and the processes involved are summarized in the rock cycle (Figure \(\PageIndex{3}\)). The rock cycle is driven by two forces: (1) Earth’s internal heat engine, which moves material around in the core and the mantle and leads to slow but significant changes within the crust, and (2) the hydrological cycle, which is the movement of water, ice, and air at the surface, and is powered by the sun.
However, before we begin our discussion of the rock cycle, let’s clarify a very important point: rocks and minerals are not the same thing! A mineral is a naturally occurring, inorganic, solid element or compound, with a definite composition or compositional range and a regular internal crystal structure. A mineral is a naturally occurring combination of specific elements that are arranged in a particular repeating three-dimensional structure or lattice.[1] The mineral halite is shown as an example in Figure \(\PageIndex{1}\).
In this case, atoms of sodium (Na: purple) alternate with atoms of chlorine (Cl: green) in all three dimensions, and the angles between the bonds are all 90°. Even in a tiny crystal, like the ones in your salt shaker, the lattices extend in all three directions for thousands of repetitions. Halite always has this composition and this structure.
A rock is a solid, cohesive aggregate of one or more minerals or mineral materials. The key difference between a rock and a mineral is the ‘regular internal crystal structure’ of a mineral. For example, Quartz is a mineral (SiO2), while Granite is a rock. If you were to look at a quartz crystal at the molecular level, the entire crystal would be made up of identical building blocks (SiO2 molecules). Granite, on the other hand is not homogenous. A close-up view of granite, a common rock, is shown in Figure \(\PageIndex{2}\). Although a hand-sized piece of granite may have thousands of individual mineral crystals in it, there are typically only a few different minerals, as shown here. Therefore, one chunk of a piece of granite, when viewed at the molecular level would appear very different from another chunk viewed at the molecular level because the first chunk might be a quartz crystal, while the second chunk might be another type of crystal.
Rocks are categorized by how they are formed. Rocks that are formed directly from liquid rock, or magma that wells up from deep in the earth are called igneous rocks. Rocks that form at, or just below the surface of the earth from weathered and decomposed bits of other rocks are called sedimentary rocks. Igneous or sedimentary rocks which are subjected to intense pressure or temperature (but not enough to melt them) form metamorphic rocks.
The rock cycle is still active on Earth because our core is hot enough to keep the mantle moving, our atmosphere is relatively thick, and we have liquid water (Figure \(\PageIndex{3}\)). On some other planets or their satellites, such as the Moon, the rock cycle is virtually dead because the core is no longer hot enough to drive mantle convection and there is no atmosphere or liquid water.
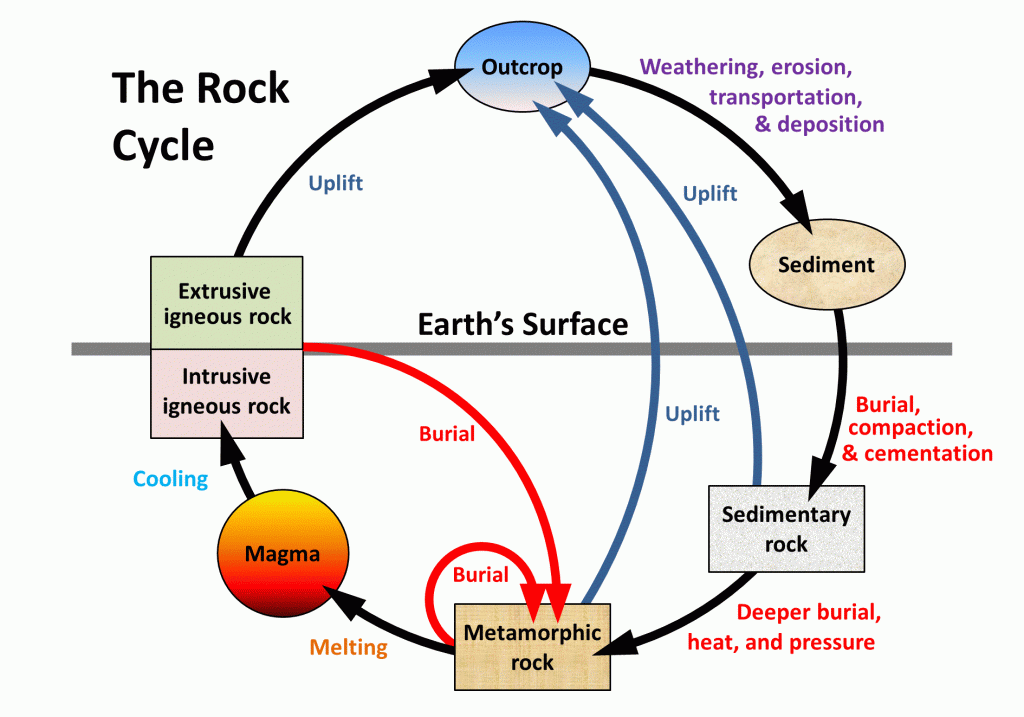
In describing the rock cycle, we can start anywhere we like, although it’s convenient to start with magma. As we’ll see in more detail below, magma is rock that is hot to the point of being entirely molten, with a temperature of between about 800° and 1300°C, depending on the composition and the pressure.

Magma can either cool slowly within the crust (over centuries to millions of years)—forming intrusive igneous rock, or erupt onto the surface and cool quickly (within seconds to years)—forming extrusive igneous rock (volcanic rock) (Figure \(\PageIndex{4}\)). Intrusive igneous rock typically crystallizes at depths of hundreds of meters to tens of kilometers below the surface. The size of crystals in igneous rocks is inversely related to the speed at which the rock cooled. Therefore, extrusive igneous rocks have very small crystals or no crystals at all because they cooled so rapidly. Basalt is a common example of an extrusive igneous rock. Because intrusive ingeneous rocks cool very slowly large (1 cm to 1 m) crystals can form. Granite is an example of an intrusive igneous rock. Most of the Sierra Nevada and Cascade mountain ranges are made up of granite. To change its position in the rock cycle, intrusive igneous rock has to be uplifted and then exposed by the erosion of the overlying rocks.
Through the various plate-tectonics-related processes of mountain building, all types of rocks are uplifted and exposed at the surface. Once exposed, they are weathered, both physically (by mechanical breaking of the rock) and chemically (by weathering of the minerals), and the weathering products—mostly small rock and mineral fragments—are eroded, transported, and then deposited as sediments. Sediment is a loose term for anything ranging from fist-sized chunks of rock in a stream bed to gravel, sand and mud. Dead organic material is also sediment. Basically, all the unconsolidated material you see at the surface of the earth is sediment. Transportation and deposition of sediments occur through the action of glaciers, streams, waves, wind, and other agents, and sediments are deposited in rivers, lakes, deserts, and the ocean.
Unless they are re-eroded and moved along, sediments will eventually be buried by more sediments. At depths of hundreds of meters or more, they become compressed and cemented into sedimentary rock (See Figure \(\PageIndex{5}\) for example). There are two types of sedimentary rocks. The first is the clastic sedimentary rock. Over time (geologic time) new sediment covers up old sediment, and there is enough pressure on the sediment to lithify it. Lithification is the process of compaction, cementation and hardening of sediments into sedimentary rocks. Clastic sedimentary rocks are composed of fragments of other rocks. They are often stacked in distinct layers with the oldest material on the bottom. An example of a common clastic sedimentary rock is sandstone, such as the sandstone layers of the Grand Canyon. The second type of sedimentary rock, the chemical sedimentary rock, forms when water seeps through sediments and dissolves certain minerals, then deposits the minerals in a new location. An example of this is deposits of gypsum or halite (table salt) which often form in the desert after rain. Another example of a chemical sedimentary rock is limestone. Limestone is precipitated calcium carbonate (CaCO3). Limestone is the primary component of much of the central United States. One problem with chemical sedimentary rocks is that it is very easy for water to re-dissolve them. Sink holes form where water has dissolved limestone in the ground, forming a cave which then collapses. Sink holes are a common hazard of areas that have thick limestone deposits.

Again through various means, largely resulting from plate-tectonic forces, different kinds of rocks are either uplifted, to be re-eroded, or buried deeper within the crust where they are heated up, squeezed, and changed into metamorphic rock (Figure \(\PageIndex{6}\)). Metamorphic rocks have been subjected to such severe pressures and/or temperatures that the chemical elements that make up the minerals in the rock rearrange themselves to form new minerals. Metamorphic rocks reach the surface the same way that intrusive igneous rocks reach the surface, through uplift, weathering and erosion. Metamorphism (the process that creates metamorphic rocks) also changes the appearance of landscapes. You can often see twisted and folded layers of rocks in metamorphic regions. Gneiss, for example, is a metamorphic rock derived from granite, while marble is derived from limestone, and slate from shale.

Contributors and Attributions
Modified by Kyle Whittinghill from the following sources
- The Rock Cycle and Minerals and Rocks from Physical Geology by Steven Earle (licensed under a Creative Commons Attribution 4.0 International License)
- The Rock Cycle by K. Allison Lenkeit-Meezan (Foothill College)
- The Physical World from Environmental Science: A Canadian Perspective by Bill Freedman (Creative Commons Attribution NonCommercial)


