7.3: Lab Procedures- Biochemical Tests
- Page ID
- 52256
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Make sure you follow aseptic procedures and label everything carefully! Use the inoculation method indicated for each type of medium—these methods may differ. Make sure to thoroughly sterilize your loop or needle between inoculations to ensure that you are only introducing one bacterial species into your medium. If a medium is inoculated with more than one kind of bacterium, a positive result cannot be attributed to a single bacterial species. Be sure to use the correct bacterial species for each test. Follow directions carefully so that you do not waste media.
For comparison purposes, you will be provided with negative controls (media that have not been inoculated) in the next lab when you are analyzing your results.
Note
All media that you inoculate today will be incubated until the next lab, when you will analyze your results .
A. Carbohydrate Fermentation
Each student: 1 tube each of the following broths: Lactose + phenol red (green cap), Sucrose + phenol red (yellow cap), Glucose + phenol red (red cap)
Instructions: Choose 1 of the following bacteria: Proteus vulgaris, Escherichia coli, Bacillis subtilis, or Streptococcus faecalis (each person at the table should choose a different species)
Inoculate the 3 types of fermentation broth with your chosen bacteria. Prior to inoculating the broths, make note of any small bubbles that might be present in the Durham tubes, so these are not read as evidence of gas formation during fermentation.
B. Triple sugar iron (TSI) slant/deep
It tests the ability of bacteria to ferment sugars and to produce H2S, often used to identify Salmonella and Shigella. The medium contains sugars (lactose, sucrose, and glucose) and thiosulfate. Slant/deep allows for aerobic and anaerobic growth conditions.

1. Inoculate your TSI slant/deep with your assigned bacteria using a needle by stabbing fully into the butt ONCE and only ONCE.
2. Then, streak across the slant WITHOUT re-dipping your needle in your plate. KEEP CAPS LOOSE and place it on the rack at the end of the table to be incubated at 37°C
C. Casein Hydrolysis
Each student: 1 milk agar plate
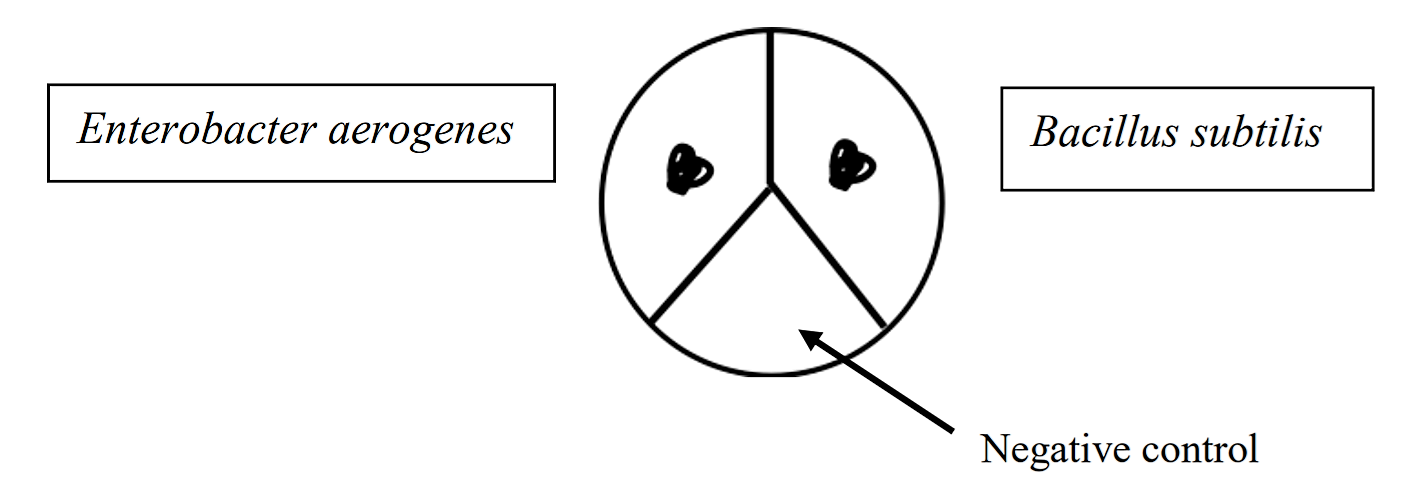
Instructions: Use both these bacteria: Bacillus subtilis and Enterobacter aerogenes
Divide your milk agar plate into 3 areas using a wax pencil or a sharpie marker. Label the plate to indicate which bacterium will be inoculated into each area. One area is left as a negative control. Using a loop, you will do spot inoculations of each bacterial species in the areas, just as you did on the starch agar plate.
.png?revision=1&size=bestfit&width=577&height=205)
D. Catalase Activity
Procedure:
1. Transfer a loopful of cells of the species to be tested onto a microscope slide. Bacillus megaterium should be used as a positive control and your unknown should be tested.
2. Add a drop of hydrogen peroxide.
3. Look closely for the evolution of bubbles.
E. Oxidase test
Procedure for BD BBL DrySlide: (each pair of students need 1 slide)
1. Open the BD BBL DrySlide Oxidase pouch and remove a slide by grabbing the edge of the slide, do not grab paper slide areas. After removing a slide, fold the top of the pouch over and seal tightly with a self-adhesive sticker (provided).
2. Using a sterile toothpick, pick a portion of the colony to be tested and apply the growth on ONE quadrant of the dry slide. To ensure a proper reaction, spread the inoculum on the slide reaction area to a 3-4mm size. Roll the toothpick over the slide reaction area to transfer the cells.
3. On one oxidase slide, there are 4 quadrants: perform this test on Bacillus megaterium as a negative control, Pseudomonas fluorescens as a positive control, your unknown, and your partner’s unknown. Examine the reaction area for appearance of a dark purple/blue color within 20sec. Disregard color development after 20 sec.
For Gibson Bioscience Swabs: (1 swab per sample used)
1. Open the Gibson Bioscience Swabs package, ensuring that you open only at the end where you can grab the handle of the swab. Touch the swab to a (control(s) or unknown sample) colony on your plate. Examine for purple/blue coloration after 10 sec. Disregard any color development occurring after 60 sec.
F. SIM Tubes
Procedure:
1. Label SIM tubes; one for Klebsiella aerogenes, one for Staphylococcus epidermidis, one for Proteus vulgaris, and one for your unknown.
2. Using your loop, inoculate the tubes with the appropriate cultures.
To inoculate, stab the center of the agar deep continuing down into the agar about 3/4 of the way to the bottom.
3. Incubate at 30°C for 48 hours (your TA will remove from incubator).
4. Following incubation, FIRST observe results for motility and sulfide production, THEN test each tube for the presence of indole by adding five drops of Kovac's reagent.
Contributions and Attributions
1. Contributed by Nazzy Pakpour & Sharon Horgan Assistant Professor (Biological Sciences) at California State University


