7.2: Introduction to Biochemical Tests Part II
- Page ID
- 52338
Learning Outcomes
- Observe and interpret the reactions of catalase positive and catalase negative bacteria using catalase reagent, explain the function of the enzyme catalase in cells.
- Observe and interpret the reactions of oxidase positive and oxidase negative bacteria using the oxidase reagent, discuss the role of the enzyme cytochrome c oxidase and respiratory pigment cytochrome c in cell respiration.
- Observe reactions of bacteria in SIM/Sulfur Indole Motility media, describe the purpose of critical ingredients in SIM media, Interpret SIM reactions
A. Catalase Activity
Byproducts of aerobic metabolism include two toxic compounds: superoxide free radicals (O2 -) and hydrogen peroxide (H2O2). These toxic compounds can cause intracellular damage, such as damage to DNA, lipids, and proteins. To remove these compounds, cells produce enzymes to break them down. Cells can convert superoxide free radicals to hydrogen peroxide by using the enzyme superoxide dismutase (SOD); catalase breaks down hydrogen peroxide into water and oxygen.
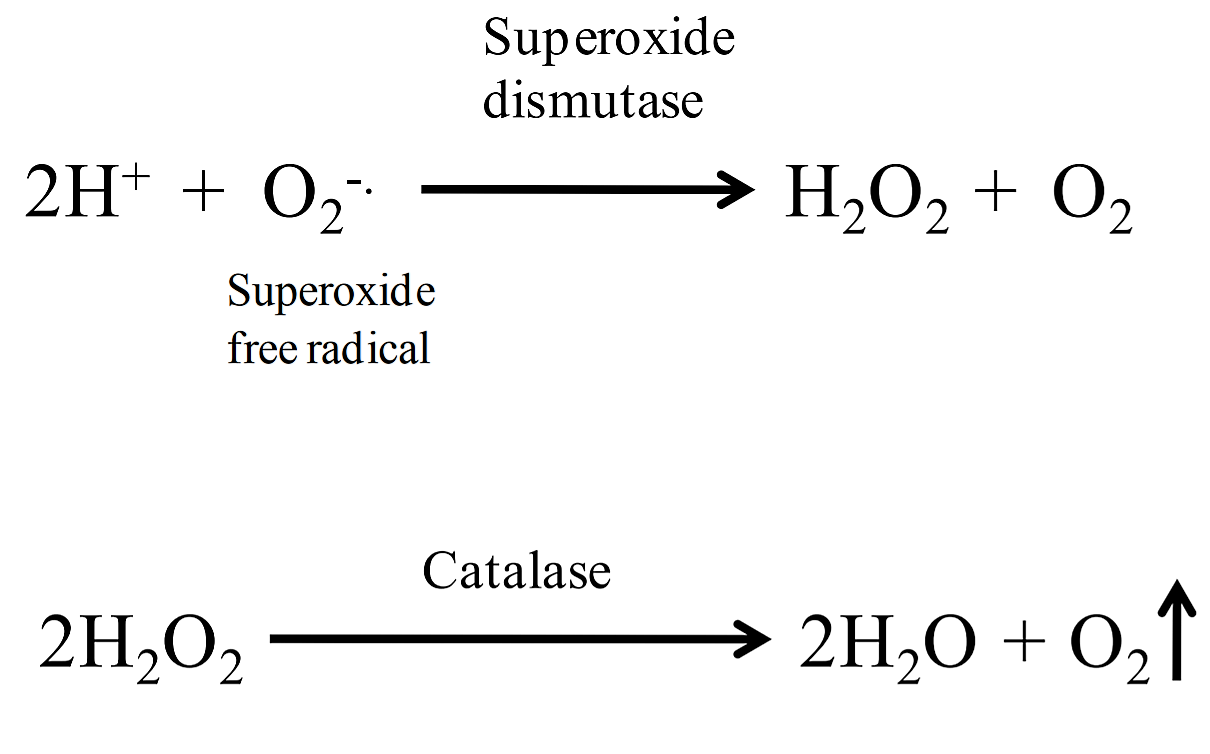.png?revision=1)
Figure 1: Superoxide dismutase and Catalase reactions
A simple test to determine if bacteria produce catalase is to add hydrogen peroxide to bacteria on an agar slant or to bacteria spread on a slide (image 1). If catalase is present, the hydrogen peroxide will be broken down into water and oxygen gas, resulting in the production of bubbles (+ test). This test does not require any special type of medium, however it should never be performed on organisms that have been grown on blood agar (a medium that contains blood). This is because there is a catalase activity in blood that would produce a false positive result. Most aerobic and facultatively anaerobic organisms produce SOD and catalase (note: some species use peroxidase rather than catalase to break down hydrogen peroxide). Obligate anaerobes lack these enzymes, which is why they cannot survive in an atmosphere containing oxygen. However, some of them have modified versions of these enzymes to deal with any possible exposure to oxygen. The archaeon, Pyrococcus furiosus, is an obligate anaerobe that lives on and near hydrothermal vents. Certain segments of these habitats are anoxic and P. furiosus occupies these niches. Some anaerobes have a superoxide-reducing system based on a different enzyme, called superoxide reductase (SOR), which reduces O2− rather than dismutating it.
Image 1: Slide catalase test results. Hydrogen peroxide was added directly to the culture on a microscope slide. A positive reaction produced by Staphylococcus aureus is indicated by bubbling; a negative reaction produced by Streptococcus pyogenes is indicated by lack of bubbling. Image by Karen Reiner, Andrews University, Berrien Springs, MI.
Watch Video 1: Catalase test
Watch video 1: Catalase test. (1:08) Video by MCCC Microbiology. URL:https://youtu.be/r3p4jHjw9o8
B. Oxidase test
Cytochrome oxidase, also known as complex IV, is the terminal, or final, enzyme of the electron transport system/ETS (this does not include ATP synthase). Cytochrome oxidase is a transmembrane molecule found in the mitochondria of eukaryotes and in the cellular space of aerobic prokaryotes. This molecule is a proton pump that plays a vital role in producing energy, in the form of ATP, via the ETS. In the last steps of the energy production process, cytochrome oxidase oxidizes the waste products from the end of the energy making process, converting reactive species, H+ and dioxygen (O2), to a more stable molecule, water (H2O).
The oxidase test is a key test to differentiate between the families of Pseudomonadaceae (ox +) and Enterobacteriaceae (ox -), and is useful for speciation and identification of many other bacteria, those that have to use oxygen as the final electron acceptor in aerobic respiration. The enzyme cytochrome oxidase is involved with the reduction of oxygen at the end of the electron transport chain.
A cytochrome c oxidase test utilizes a special reagent called oxidase reagent, which is a 1% solution of the chemical tetramethyl-para-phenylenediamine (TMDPD) dihydrochloride. The reduced form of this reagent is colorless, but donates electrons to the cell’s cytochrome c oxidase forming an oxidized colored form.
- Oxidase positive bacteria change the color of the reagent from colorless to colored and finally black.
- Oxidase negative bacteria do not contain the cytochrome c oxidase and do not change the color of oxidase reagent from colorless to black.
There are two ways to do the oxidase test, one is using a filter paper (see image 2 below) and the oxidase reagent and the second is doing a ‘plate’ oxidase test (image 3).
Image 2: Oxidase test on filter paper. A positive oxidase result given by Pseudomonas aeruginosa (left) is indicated by a purple color. A negative oxidase result given by Escherichia coli (right) is indicated by the lack of color change. Both organisms were rubbed onto a filter that was dipped in oxidase reagent and allowed to dry. Image by Laura Cathcart, University of Maryland, College Park, MD; Sabrina Kramer, University of Maryland, College Park, MD; Patricia Shields, University of Maryland, College Park, MD.
Image 3: Oxidase test on an agar plate with bacterial colonies. This close up view of an agar plate has a mixed culture of oxidase-positive Vibrio cholerae, indicated by purple colonies, and oxidase-negative Escherichia coli, indicated by lack of color change (they are the white colonies), demonstrate how the plate oxidase test differentiates between the two. In this test, the oxidase reagent was added directly to the colonies which were grown on trypticase soy agar at 37°C for 24 hours. Image by Laura Cathcart, University of Maryland, College Park, MD; Sabrina Kramer, University of Maryland, College Park, MD; Patricia Shields, University of Maryland, College Park, MD.
Oxidase test is most helpful in screening colonies suspected of being one of the Enterobacteriaceae (all negative) and in identifying colonies suspected of belonging to other genera such as Aeromonas, Pseudomonas, Neisseria, Campylobacter, and Pasteurella (positive).
Watch Video 2: Oxidase Test
Watch Video 2: how to perform an oxidase test. (3:23) Video by URMICRO1. URL: https://youtu.be/7Aa1xO2cC1M
C. Sulfide Indole Motility (SIM) Medium
The Sulfide-Indole-Motility (SIM) medium was devised for use as a routine medium in the cultural identification of members of the Salmonella and Shigella groups, showing hydrogen sulfide production, indole production, and motility in the same tube. These characteristics, along with other biochemical reactions, are of prime importance in the clinical identification of members of the Gram-negative enteric group, many of which are bacterial pathogens. This enteric group is named after the Greek word for intestine - enteron.
Sulfide production is tested by the presence of a black precipitate. Microorganisms are capable of producing sulfide and hydrogen sulfide in several ways. One way is when sulfur containing organic compounds, such as the amino acid cysteine, are metabolized, they excrete the sulfide ion (S2-) or hydrogen sulfide (H2S) as a waste product, which can then combine with the ferrous iron (Fe2+) that is present in the medium to produce ferrous sulfide, which appears as a black precipitate. Bacteria capable of producing the enzyme cysteine desulfhydrase are able to remove the sulfhydryl group (- SH) from cysteine to produce sulfide ions (which may combine with hydrogen ions to produce hydrogen sulfide), ammonia and pyruvic acid.
Figure 2: Chemical reaction of cysteine desulfhydrase and formation of ferrous sulfide
Another common way for bacteria to produce hydrogen sulfide is through the use of sulfate (SO42-) as a terminal electron acceptor. Anaerobic respiration is the use of an inorganic compound (other than oxygen) as a terminal electron acceptor. You will recall that in aerobic respiration, O2 is the final electron acceptor, and H2O is produced as an end product. In anaerobic respiration, SO42- would be reduced to H2S. Anaerobic respiration most commonly involves NO3-, SO32-, and CO32-. When the bacteria produce hydrogen sulfide, it reacts with ferrous ions present in the culture medium to form the insoluble, black iron/ferrous sulfide precipitate.
Indole production is indicative of the intracellular enzyme tryptophanase. Tryptophanase is an intracellular enzyme, which catalyzes the breakdown of the amino acid tryptophan to pyruvate and indole. The pyruvate is used by the cell as a carbon and energy source; the indole is excreted as a waste product. To test for tryptophanase, a culture is grown in the SIM medium, which contains high levels of tryptophan. After incubation and growth, several drops of Kovac's reagent are added. Kovac's reagent contains para-dimethyl-aminobenzaldehyde, which reacts with indole to produce a reddish pink compound (rosindole). The development of a red ring at the top of the tube is a positive test, see Image 2, tube A and E.
Motility is tested by growth in a semi-solid, soft agar. Motile bacteria can swim through the medium and will show diffuse growth and turbidity away from the line of inoculation.
Image 3: Sulfur-indole-motility (SIM) test results from various microbes. From left to right: (A) Escherichia coli, (B) Staphylococcus aureus, (C) Salmonella arizonae, (D) Enterobacter aerogenes, and (E) Proteus vulgaris. After addition of Kovács reagent, a pink ring at the top of the tube indicates a positive indole result (A and E). Blackening of the media indicates hydrogen sulfide production (C and E). Growth feathering away from the stab line creating a cloudy appearance in the media indicates motility (A, C, D, and E). Growth strictly along the stab line indicates a nonmotile organism (B). Image by Tasha L. Sturm, Cabrillo College, Aptos, CA.
Watch Video 3: SIM medium
Watch Video 3: SIM medium inoculation and interpretation. Video by Dr. Gary Kaiser (4:30) URL: https://youtu.be/zzBhhVp0qvU
Contributors and Attributions
1. Contributed by Nazzy Pakpour & Sharon Horgan Assistant Professor (Biological Sciences) at California State University
2. Jackie Reynolds, Professor of Biology (Richland College)

