13.2: Second Line Defenses: Cells and Fluids
- Page ID
- 31858
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Identify and describe the components of blood
- Explain the process by which the formed elements of blood are formed (hematopoiesis)
- Describe the characteristics of formed elements found in peripheral blood, as well as their respective functions within the innate immune system
- Explain why the circulatory and lymphatic systems lack normal microbiota
Plasma Protein Mediators
Many nonspecific innate immune factors are found in plasma, the fluid portion of blood. Plasma contains electrolytes, sugars, lipids, and proteins, each of which helps to maintain homeostasis (i.e., stable internal body functioning), and contains the proteins involved in the clotting of blood. Additional proteins found in blood plasma, such as acute-phase proteins, complement proteins, and cytokines, are involved in the nonspecific innate immune response.
Plasma versus Serum
There are two terms for the fluid portion of blood: plasma and serum. How do they differ if they are both fluid and lack cells? The fluid portion of blood left over after coagulation (blood cell clotting) has taken place is serum. Although molecules such as many vitamins, electrolytes, certain sugars, complement proteins, and antibodies are still present in serum, clotting factors are largely depleted. Plasma, conversely, still contains all the clotting elements. To obtain plasma from blood, an anticoagulant must be used to prevent clotting. Examples of anticoagulants include heparin and ethylene diamine tetraacetic acid (EDTA). Because clotting is inhibited, once obtained, the sample must be gently spun down in a centrifuge. The heavier, denser blood cells form a pellet at the bottom of a centrifuge tube, while the fluid plasma portion, which is lighter and less dense, remains above the cell pellet.
Acute-Phase Proteins
The acute-phase proteins are another class of antimicrobial mediators. Acute-phase proteins are primarily produced in the liver and secreted into the blood in response to inflammatory molecules from the immune system. Examples of acute-phase proteins include C-reactive protein, serum amyloid A, ferritin, transferrin, fibrinogen, and mannose-binding lectin. Each of these proteins has a different chemical structure and inhibits or destroys microbes in some way (Table \(\PageIndex{1}\)).
Table \(\PageIndex{1}\): Some Acute-Phase Proteins and Their Functions
| Some Acute-Phase Proteins and Their Functions | |
|---|---|
| C-reactive protein | Coats bacteria (opsonization), preparing them for ingestion by phagocytes |
| Serum amyloid A | |
| Ferritin | Bind and sequester iron, thereby inhibiting the growth of pathogens |
| Transferrin | |
| Fibrinogen | Involved in formation of blood clots that trap bacterial pathogens |
| Mannose-binding lectin | Activates complement cascade |
The Complement System
The complement system is a group of plasma protein mediators that can act as an innate nonspecific defense while also serving to connect innate and adaptive immunity (discussed in the next chapter). The complement system is composed of more than 30 proteins (including C1 through C9) that normally circulate as precursor proteins in blood. These precursor proteins become activated when stimulated or triggered by a variety of factors, including the presence of microorganisms. Complement proteins are considered part of innate nonspecific immunity because they are always present in the blood and tissue fluids, allowing them to be activated quickly. Also, when activated through the alternative pathway (described later in this section), complement proteins target pathogens in a nonspecific manner. The process by which circulating complement precursors become functional is called complement activation. This process is a cascade that can be triggered by one of three different mechanisms, known as the alternative, classical, and lectin pathways.
The alternative pathway is initiated by the spontaneous activation of the complement protein C3. The hydrolysis of C3 produces two products, C3a and C3b. When no invader microbes are present, C3b is very quickly degraded in a hydrolysis reaction using the water in the blood. However, if invading microbes are present, C3b attaches to the surface of these microbes. Once attached, C3b will recruit other complement proteins in a cascade (Figure \(\PageIndex{1}\)).
The classical pathway provides a more efficient mechanism of activating the complement cascade, but it depends upon the production of antibodies by the specific adaptive immune defenses. To initiate the classical pathway, a specific antibody must first bind to the pathogen to form an antibody-antigen complex. This activates the first protein in the complement cascade, the C1 complex. The C1 complex is a multipart protein complex, and each component participates in the full activation of the overall complex. Following recruitment and activation of the C1 complex, the remaining classical pathway complement proteins are recruited and activated in a cascading sequence (Figure \(\PageIndex{1}\)).
The lectin activation pathway is similar to the classical pathway, but it is triggered by the binding of mannose-binding lectin, an acute-phase protein, to carbohydrates on the microbial surface. Like other acute-phase proteins, lectins are produced by liver cells and are commonly upregulated in response to inflammatory signals received by the body during an infection (Figure \(\PageIndex{1}\)).
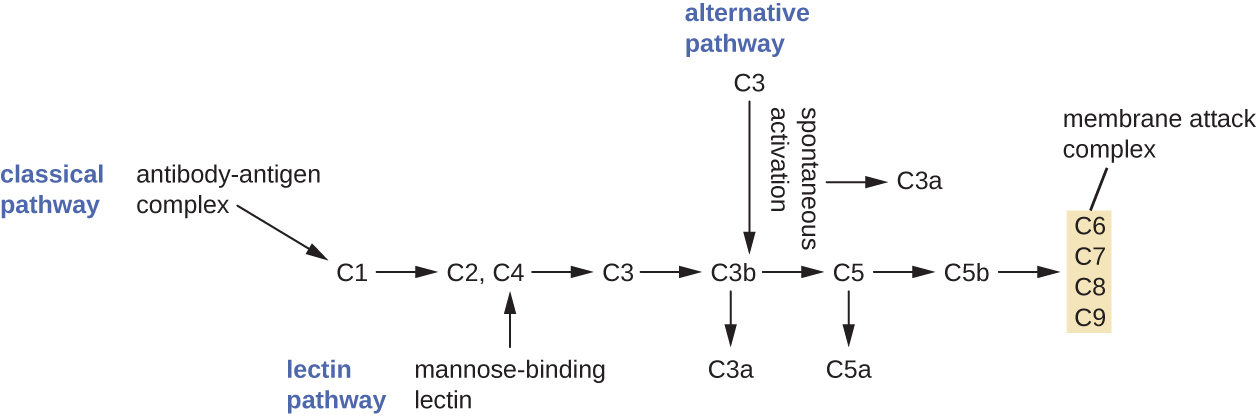
Although each complement activation pathway is initiated in a different way, they all provide the same protective outcomes: opsonization, inflammation, chemotaxis, and cytolysis. The term opsonization refers to the coating of a pathogen by a chemical substance (called an opsonin) that allows phagocytic cells to recognize, engulf, and destroy it more easily. Opsonins from the complement cascade include C1q, C3b, and C4b. Additional important opsonins include mannose-binding proteins and antibodies. The complement fragments C3a and C5a are well-characterized anaphylatoxins with potent proinflammatory functions. Anaphylatoxins activate mast cells, causing degranulation and the release of inflammatory chemical signals, including mediators that cause vasodilation and increased vascular permeability. C5a is also one of the most potent chemoattractants for neutrophils and other white blood cells, cellular defenses that will be discussed in the next section.
The complement proteins C6, C7, C8, and C9 assemble into a membrane attack complex (MAC), which allows C9 to polymerize into pores in the membranes of gram-negative bacteria. These pores allow water, ions, and other molecules to move freely in and out of the targeted cells, eventually leading to cell lysis and death of the pathogen (Figure \(\PageIndex{1}\)). However, the MAC is only effective against gram-negative bacteria; it cannot penetrate the thick layer of peptidoglycan associated with cell walls of gram-positive bacteria. Since the MAC does not pose a lethal threat to gram-positive bacterial pathogens, complement-mediated opsonization is more important for their clearance.
Cytokines
Cytokines are soluble proteins that act as communication signals between cells. In a nonspecific innate immune response, various cytokines may be released to stimulate production of chemical mediators or other cell functions, such as cell proliferation, cell differentiation, inhibition of cell division, apoptosis, and chemotaxis.
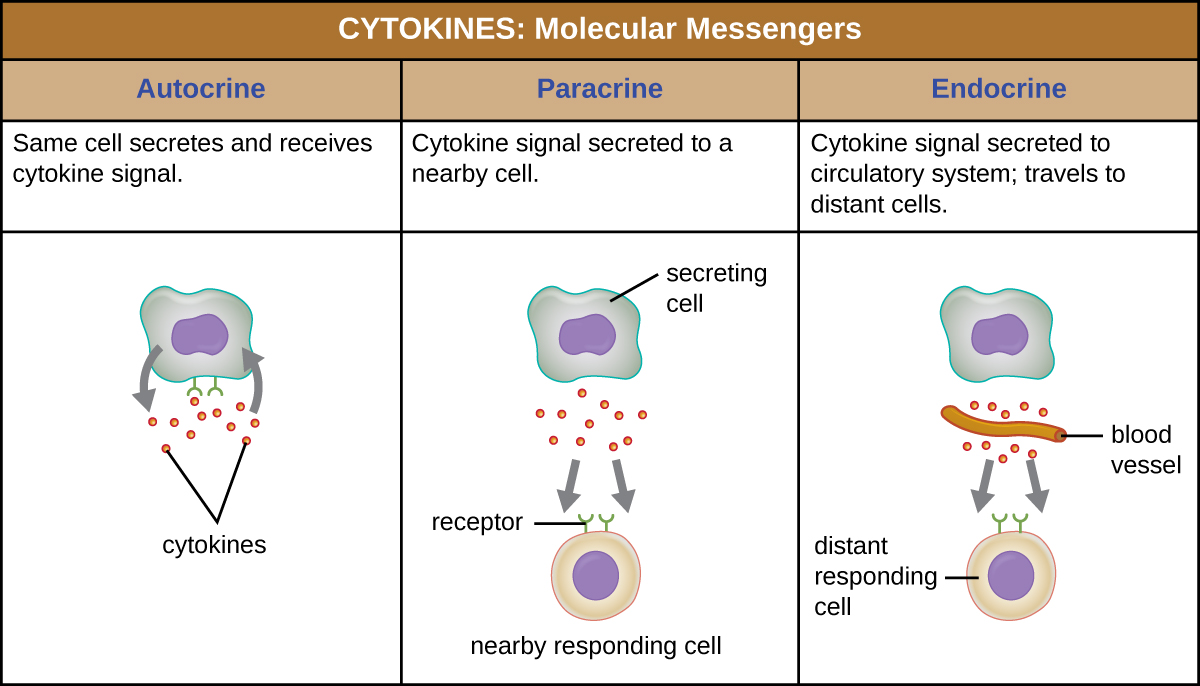
When a cytokine binds to its target receptor, the effect can vary widely depending on the type of cytokine and the type of cell or receptor to which it has bound. The function of a particular cytokine can be described as autocrine, paracrine, or endocrine (Figure \(\PageIndex{2}\)). In autocrine function, the same cell that releases the cytokine is the recipient of the signal; in other words, autocrine function is a form of self-stimulation by a cell. In contrast, paracrine function involves the release of cytokines from one cell to other nearby cells, stimulating some response from the recipient cells. Last, endocrine function occurs when cells release cytokines into the bloodstream to be carried to target cells much farther away.
Three important classes of cytokines are the interleukins, chemokines, and interferons. The interleukins were originally thought to be produced only by leukocytes (white blood cells) and to only stimulate leukocytes, thus the reasons for their name. Although interleukins are involved in modulating almost every function of the immune system, their role in the body is not restricted to immunity. Interleukins are also produced by and stimulate a variety of cells unrelated to immune defenses.
The chemokines are chemotactic factors that recruit leukocytes to sites of infection, tissue damage, and inflammation. In contrast to more general chemotactic factors, like complement factor C5a, chemokines are very specific in the subsets of leukocytes they recruit.
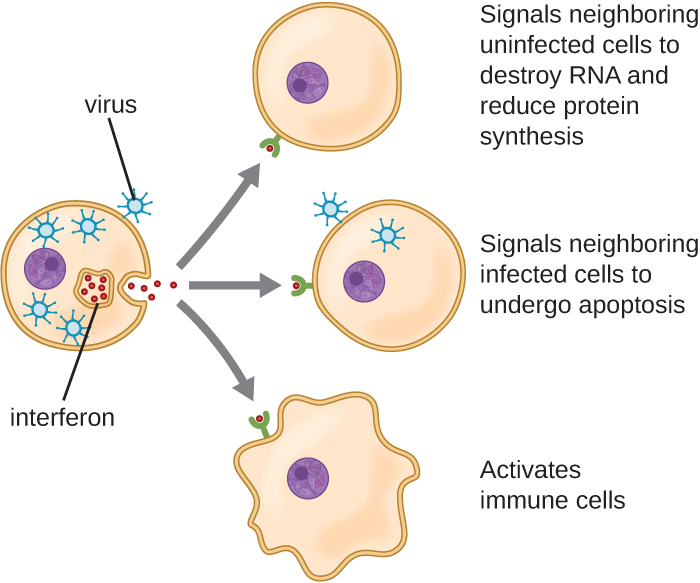
Interferons are a diverse group of immune signaling molecules and are especially important in our defense against viruses. Type I interferons (interferon-α and interferon-β) are produced and released by cells infected with virus. These interferons stimulate nearby cells to stop production of mRNA, destroy RNA already produced, and reduce protein synthesis. These cellular changes inhibit viral replication and production of mature virus, slowing the spread of the virus. Type I interferons also stimulate various immune cells involved in viral clearance to more aggressively attack virus-infected cells. Type II interferon (interferon-γ) is an important activator of immune cells (Figure \(\PageIndex{3}\)).
Inflammation-Eliciting Mediators
Many of the chemical mediators discussed in this section contribute in some way to inflammation and fever, which are nonspecific immune responses. Cytokines stimulate the production of acute-phase proteins such as C-reactive protein and mannose-binding lectin in the liver. These acute-phase proteins act as opsonins, activating complement cascades through the lectin pathway.
Some cytokines also bind mast cells and basophils, inducing them to release histamine, a proinflammatory compound. Histamine receptors are found on a variety of cells and mediate proinflammatory events, such as bronchoconstriction (tightening of the airways) and smooth muscle contraction.
In addition to histamine, mast cells may release other chemical mediators, such as leukotrienes. Leukotrienes are lipid-based proinflammatory mediators that are produced from the metabolism of arachidonic acid in the cell membrane of leukocytes and tissue cells. Compared with the proinflammatory effects of histamine, those of leukotrienes are more potent and longer lasting. Together, these chemical mediators can induce coughing, vomiting, and diarrhea, which serve to expel pathogens from the body.
Certain cytokines also stimulate the production of prostaglandins, chemical mediators that promote the inflammatory effects of kinins and histamines. Prostaglandins can also help to set the body temperature higher, leading to fever, which promotes the activities of white blood cells and slightly inhibits the growth of pathogenic microbes.
Another inflammatory mediator, bradykinin, contributes to edema, which occurs when fluids and leukocytes leak out of the bloodstream and into tissues. It binds to receptors on cells in the capillary walls, causing the capillaries to dilate and become more permeable to fluids.
Exercise \(\PageIndex{1}\)
- What do the three complement activation pathways have in common?
- Explain autocrine, paracrine, and endocrine signals.
- Name two important inflammation-eliciting mediators.
Non-specific Cells
The non-fluid portion of blood consists of various types of formed elements, so called because they are all formed from the same stem cells found in bone marrow. The three major categories of formed elements are: red blood cells (RBCs), also called erythrocytes; platelets, also called thrombocytes; and white blood cells (WBCs), also called leukocytes.
Red blood cells are primarily responsible for carrying oxygen to tissues. Platelets are cellular fragments that participate in blood clot formation and tissue repair. Several different types of WBCs participate in various nonspecific mechanisms of innate and adaptive immunity. In this section, we will focus primarily on the innate mechanisms of various types of WBCs.
Hematopoiesis
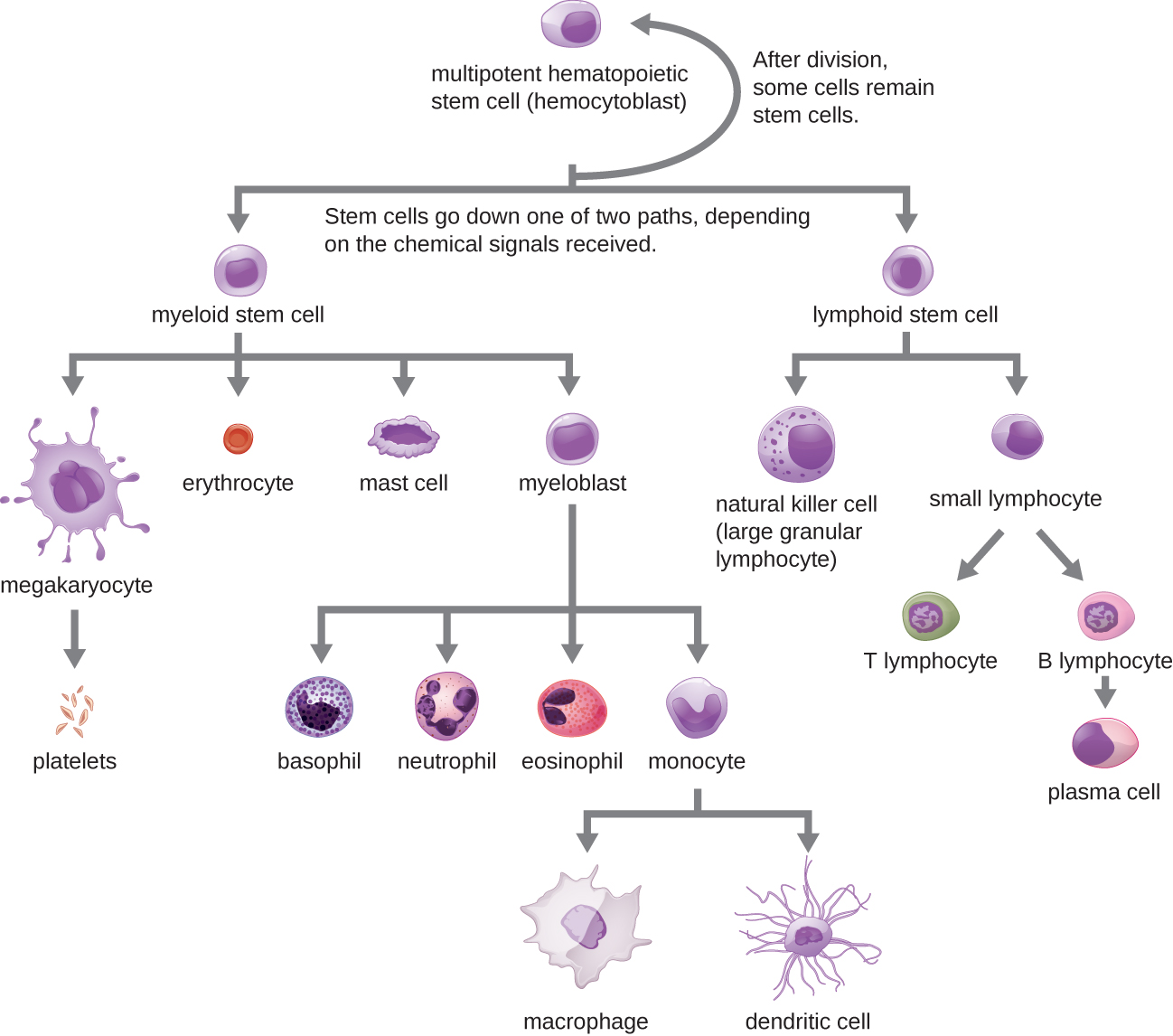
All of the formed elements of blood are derived from pluripotent hematopoietic stem cells (HSCs) in the bone marrow. As the HSCs make copies of themselves in the bone marrow, individual cells receive different cues from the body that control how they develop and mature. As a result, the HSCs differentiate into different types of blood cells that, once mature, circulate in peripheral blood. This process of differentiation, called hematopoiesis, is shown in more detail in Figure \(\PageIndex{4}\).
In terms of sheer numbers, the vast majority of HSCs become erythrocytes. Much smaller numbers become leukocytes and platelets. Leukocytes can be further subdivided into granulocytes, which are characterized by numerous granules visible in the cytoplasm, and agranulocytes, which lack granules. Figure \(\PageIndex{5}\) provides an overview of the various types of formed elements, including their relative numbers, primary function, and lifespans.
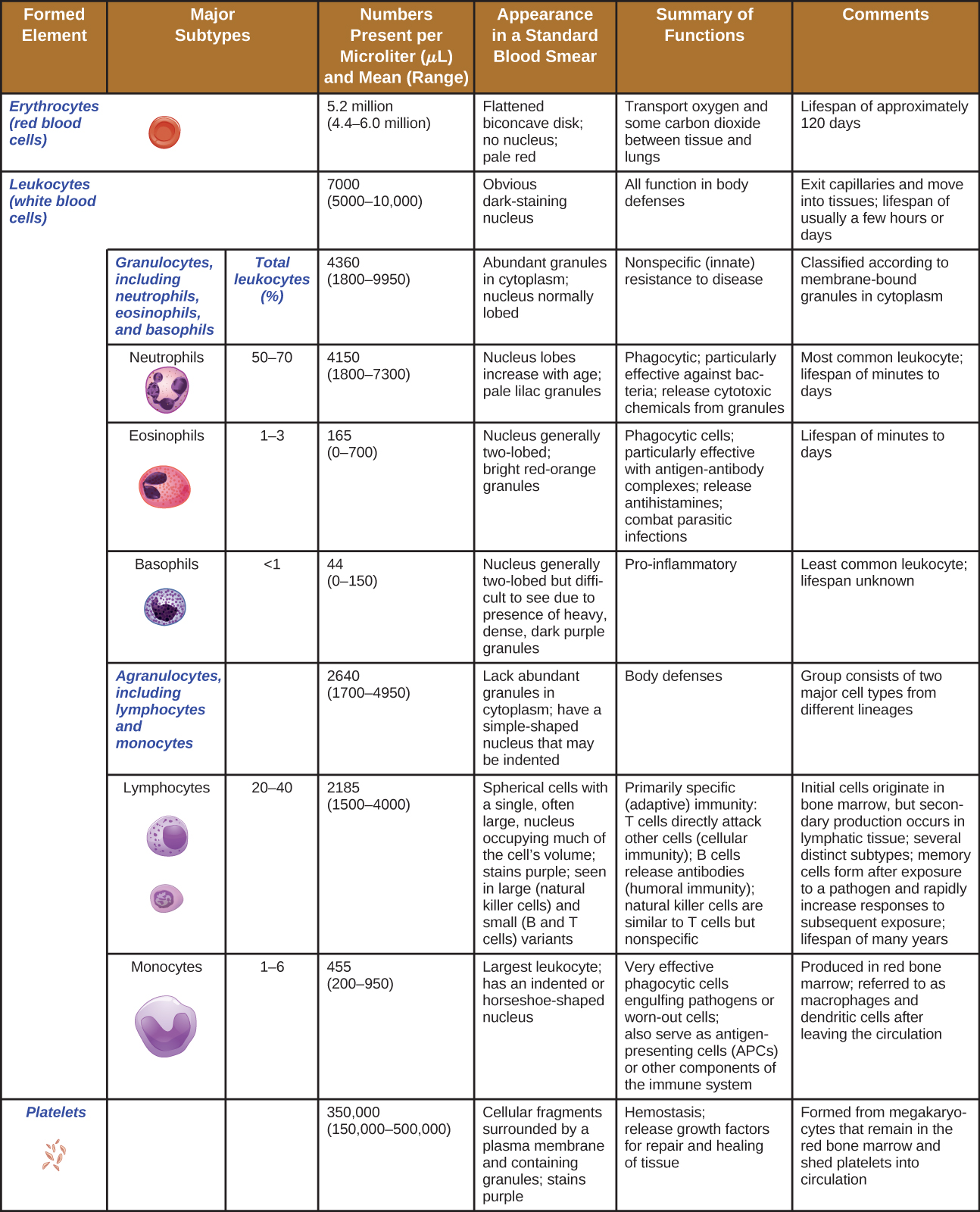
Granulocytes
The various types of granulocytes can be distinguished from one another in a blood smear by the appearance of their nuclei and the contents of their granules, which confer different traits, functions, and staining properties. The neutrophils, also called polymorphonuclear neutrophils (PMNs), have a nucleus with three to five lobes and small, numerous, lilac-colored granules. Each lobe of the nucleus is connected by a thin strand of material to the other lobes. The eosinophils have fewer lobes in the nucleus (typically 2–3) and larger granules that stain reddish-orange. The basophils have a two-lobed nucleus and large granules that stain dark blue or purple (Figure \(\PageIndex{6}\)).
Neutrophils (PMNs)
Neutrophils (PMNs) are frequently involved in the elimination and destruction of extracellular bacteria. They are capable of migrating through the walls of blood vessels to areas of bacterial infection and tissue damage, where they seek out and kill infectious bacteria. PMN granules contain a variety of defensins and hydrolytic enzymes that help them destroy bacteria through phagocytosis. In addition, when many neutrophils are brought into an infected area, they can be stimulated to release toxic molecules into the surrounding tissue to better clear infectious agents. This is called degranulation.
Another mechanism used by neutrophils is neutrophil extracellular traps (NETs), which are extruded meshes of chromatin that are closely associated with antimicrobial granule proteins and components. Chromatin is DNA with associated proteins (usually histone proteins, around which DNA wraps for organization and packing within a cell). By creating and releasing a mesh or lattice-like structure of chromatin that is coupled with antimicrobial proteins, the neutrophils can mount a highly concentrated and efficient attack against nearby pathogens. Proteins frequently associated with NETs include lactoferrin, gelatinase, cathepsin G, and myeloperoxidase. Each has a different means of promoting antimicrobial activity, helping neutrophils eliminate pathogens. The toxic proteins in NETs may kill some of the body’s own cells along with invading pathogens. However, this collateral damage can be repaired after the danger of the infection has been eliminated.
As neutrophils fight an infection, a visible accumulation of leukocytes, cellular debris, and bacteria at the site of infection can be observed. This buildup is what we call pus (also known as purulent or suppurative discharge or drainage). The presence of pus is a sign that the immune defenses have been activated against an infection; historically, some physicians believed that inducing pus formation could actually promote the healing of wounds. The practice of promoting “laudable pus” (by, for instance, wrapping a wound in greasy wool soaked in wine) dates back to the ancient physician Galen in the 2nd century AD, and was practiced in variant forms until the 17th century (though it was not universally accepted). Today, this method is no longer practiced because we now know that it is not effective. Although a small amount of pus formation can indicate a strong immune response, artificially inducing pus formation does not promote recovery.
Eosinophils
Eosinophils are granulocytes that protect against protozoa and helminths; they also play a role in allergic reactions. The granules of eosinophils, which readily absorb the acidic reddish dye eosin, contain histamine, degradative enzymes, and a compound known as major basic protein (MBP) (Figure \(\PageIndex{3}\)). MBP binds to the surface carbohydrates of parasites, and this binding is associated with disruption of the cell membrane and membrane permeability.
Basophils
Basophils have cytoplasmic granules of varied size and are named for their granules’ ability to absorb the basic dye methylene blue (Figure \(\PageIndex{3}\)). Their stimulation and degranulation can result from multiple triggering events. Activated complement fragments C3a and C5a, produced in the activation cascades of complement proteins, act as anaphylatoxins by inducing degranulation of basophils and inflammatory responses. This cell type is important in allergic reactions and other responses that involve inflammation. One of the most abundant components of basophil granules is histamine, which is released along with other chemical factors when the basophil is stimulated. These chemicals can be chemotactic and can help to open the gaps between cells in the blood vessels. Other mechanisms for basophil triggering require the assistance of antibodies.
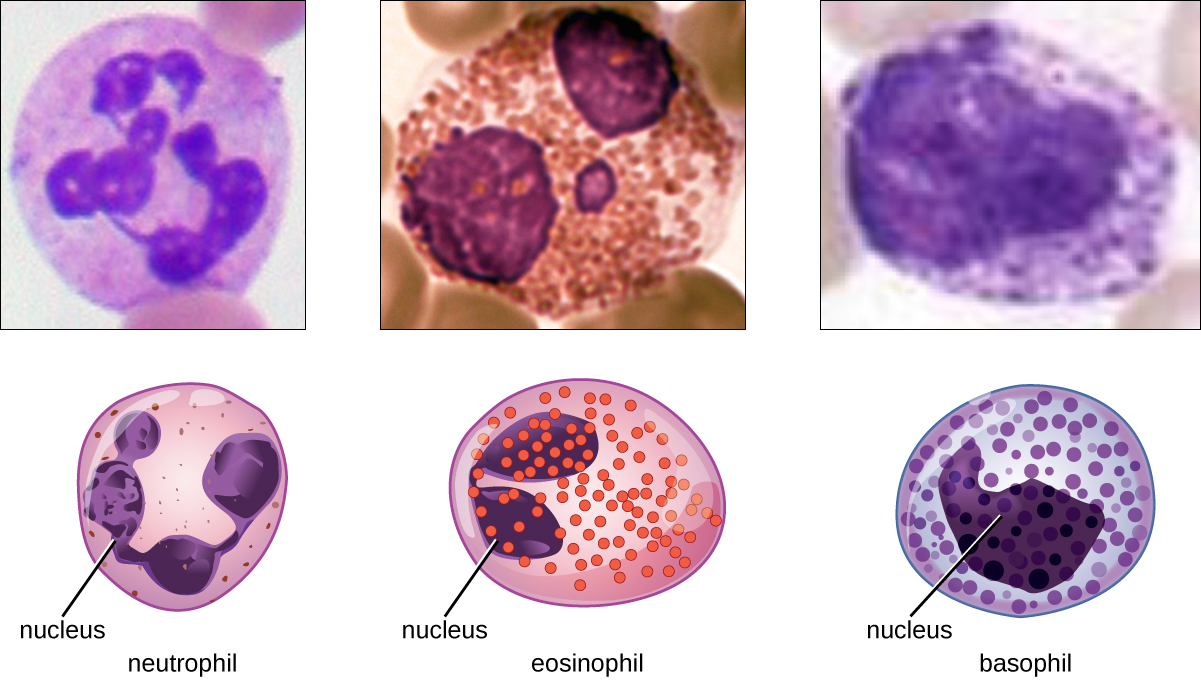
Mast Cells
Hematopoiesis also gives rise to mast cells, which appear to be derived from the same common myeloid progenitor cell as neutrophils, eosinophils, and basophils. Functionally, mast cells are very similar to basophils, containing many of the same components in their granules (e.g., histamine) and playing a similar role in allergic responses and other inflammatory reactions. However, unlike basophils, mast cells leave the circulating blood and are most frequently found residing in tissues. They are often associated with blood vessels and nerves or found close to surfaces that interface with the external environment, such as the skin and mucous membranes in various regions of the body (Figure \(\PageIndex{7}\)).
Exercise \(\PageIndex{2}\)
- Describe the granules and nuclei of neutrophils, eosinophils, basophils, and mast cells.
- Name three antimicrobial mechanisms of neutrophils
Clinical Focus: part 2
To relieve the constriction of her airways, Angela is immediately treated with antihistamines and administered corticosteroids through an inhaler, and then monitored for a period of time. Though her condition does not worsen, the drugs do not seem to be alleviating her condition. She is admitted to the hospital for further observation, testing, and treatment.
Following admission, a clinician conducts allergy testing to try to determine if something in her environment might be triggering an allergic inflammatory response. A doctor orders blood analysis to check for levels of particular cytokines. A sputum sample is also taken and sent to the lab for microbial staining, culturing, and identification of pathogens that could be causing an infection.
Exercise \(\PageIndex{3}\)
- Which aspects of the innate immune system could be contributing to Angela’s airway constriction?
- Why was Angela treated with antihistamines?
- Why would the doctor be interested in levels of cytokines in Angela’s blood?
Agranulocytes
As their name suggests, agranulocytes lack visible granules in the cytoplasm. Agranulocytes can be categorized as lymphocytes or monocytes (Figure \(\PageIndex{2}\)). Among the lymphocytes are natural killer cells, which play an important role in nonspecific innate immune defenses. Lymphocytes also include the B cells and T cells, which are discussed in the next chapter because they are central players in the specific adaptive immune defenses. The monocytes differentiate into macrophages and dendritic cells, which are collectively referred to as the mononuclear phagocyte system.
Natural Killer Cells
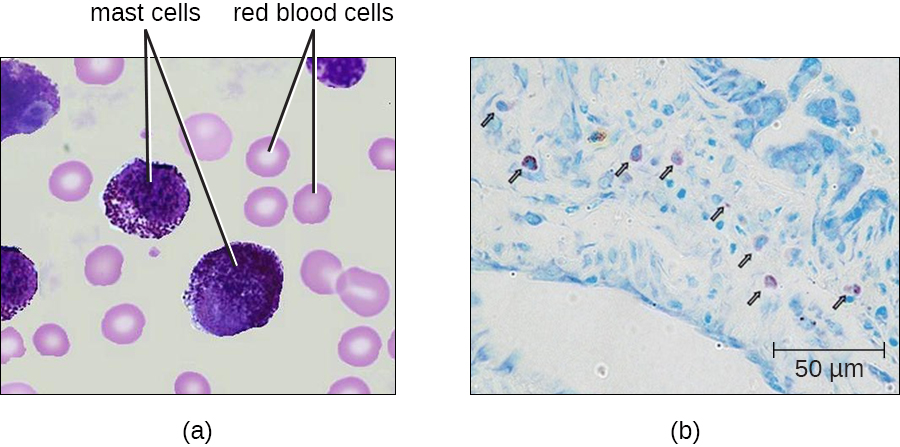
Most lymphocytes are primarily involved in the specific adaptive immune response, and thus will be discussed in the following chapter. An exception is the natural killer cells (NK cells); these mononuclear lymphocytes use nonspecific mechanisms to recognize and destroy cells that are abnormal in some way. Cancer cells and cells infected with viruses are two examples of cellular abnormalities that are targeted by NK cells. Recognition of such cells involves a complex process of identifying inhibitory and activating molecular markers on the surface of the target cell. Molecular markers that make up the major histocompatibility complex (MHC) are expressed by healthy cells as an indication of “self.” This will be covered in more detail in next chapter. NK cells are able to recognize normal MHC markers on the surface of healthy cells, and these MHC markers serve as an inhibitory signal preventing NK cell activation. However, cancer cells and virus-infected cells actively diminish or eliminate expression of MHC markers on their surface. When these MHC markers are diminished or absent, the NK cell interprets this as an abnormality and a cell in distress. This is one part of the NK cell activation process (Figure \(\PageIndex{8}\)). NK cells are also activated by binding to activating molecular molecules on the target cell. These activating molecular molecules include “altered self” or “nonself” molecules. When a NK cell recognizes a decrease in inhibitory normal MHC molecules and an increase in activating molecules on the surface of a cell, the NK cell will be activated to eliminate the cell in distress.
Once a cell has been recognized as a target, the NK cell can use several different mechanisms to kill its target. For example, it may express cytotoxic membrane proteins and cytokines that stimulate the target cell to undergo apoptosis, or controlled cell suicide. NK cells may also use perforin-mediated cytotoxicity to induce apoptosis in target cells. This mechanism relies on two toxins released from granules in the cytoplasm of the NK cell: perforin, a protein that creates pores in the target cell, and granzymes, proteases that enter through the pores into the target cell’s cytoplasm, where they trigger a cascade of protein activation that leads to apoptosis. The NK cell binds to the abnormal target cell, releases its destructive payload, and detaches from the target cell. While the target cell undergoes apoptosis, the NK cell synthesizes more perforin and proteases to use on its next target.
NK cells contain these toxic compounds in granules in their cytoplasm. When stained, the granules are azurophilic and can be visualized under a light microscope (Figure \(\PageIndex{9}\)). Even though they have granules, NK cells are not considered granulocytes because their granules are far less numerous than those found in true granulocytes. Furthermore, NK cells have a different lineage than granulocytes, arising from lymphoid rather than myeloid stem cells (Figure \(\PageIndex{9}\)).
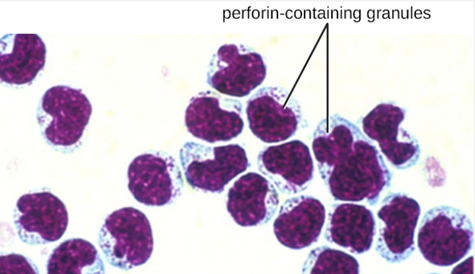
Figure \(\PageIndex{9}\): Natural killer cell with perforin-containing granules. (credit: modification of work by Rolstad B)
Monocytes
The largest of the white blood cells, monocytes have a nucleus that lacks lobes, and they also lack granules in the cytoplasm (Figure \(\PageIndex{10}\)). Nevertheless, they are effective phagocytes, engulfing pathogens and apoptotic cells to help fight infection.
When monocytes leave the bloodstream and enter a specific body tissue, they differentiate into tissue-specific phagocytes called macrophages and dendritic cells. They are particularly important residents of lymphoid tissue, as well as nonlymphoid sites and organs. Macrophages and dendritic cells can reside in body tissues for significant lengths of time. Macrophages in specific body tissues develop characteristics suited to the particular tissue. Not only do they provide immune protection for the tissue in which they reside but they also support normal function of their neighboring tissue cells through the production of cytokines. Macrophages are given tissue-specific names, and a few examples of tissue-specific macrophages are listed in Table \(\PageIndex{2}\). Dendritic cells are important sentinels residing in the skin and mucous membranes, which are portals of entry for many pathogens. Monocytes, macrophages, and dendritic cells are all highly phagocytic and important promoters of the immune response through their production and release of cytokines. These cells provide an essential bridge between innate and adaptive immune responses, as discussed in the next section as well as the next chapter.
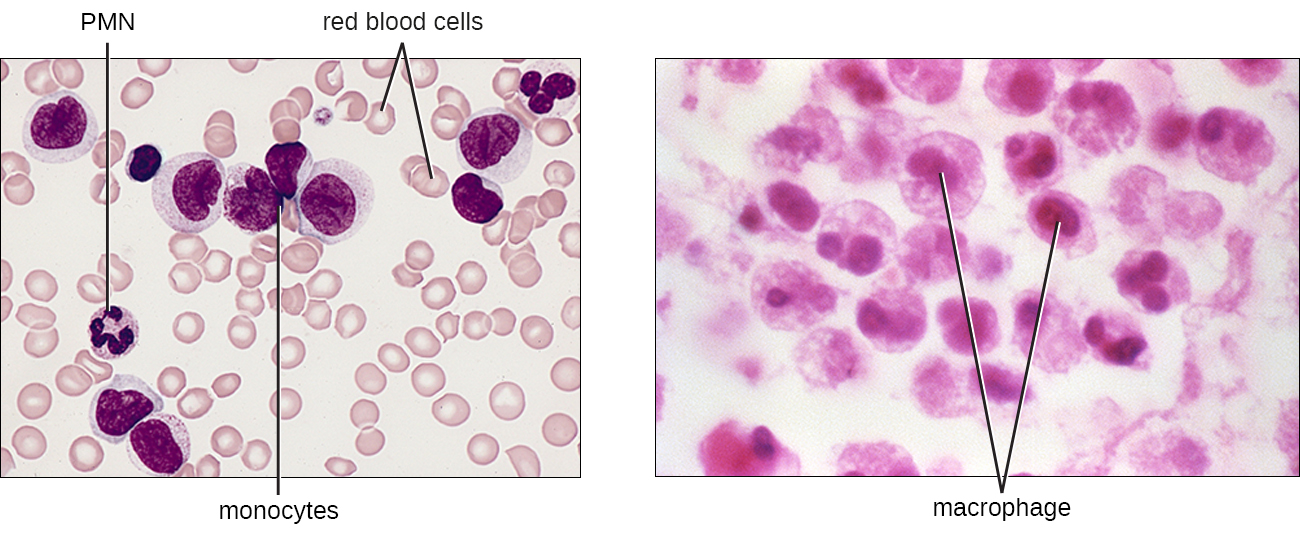
Table \(\PageIndex{2}\): Macrophages Found in Various Body Tissues
| Tissue | Macrophage |
|---|---|
| Brain and central nervous system | Microglial cells |
| Liver | Kupffer cells |
| Lungs | Alveolar macrophages (dust cells) |
| Peritoneal cavity | Peritoneal macrophages |
Exercise \(\PageIndex{4}\)
- Describe the signals that activate natural killer cells.
- What is the difference between monocytes and macrophages?
The Circulatory System
The proteins, fluids and cells of the second line defense travel via two systems. The main system being the circulatory (or cardiovascular) system is a closed network of organs and vessels that moves blood around the body (Figure \(\PageIndex{11}\)). The primary purposes of the circulatory system are to deliver nutrients, immune factors, and oxygen to tissues and to carry away waste products for elimination. The heart is a four-chambered pump that propels the blood throughout the body. When the heart contracts, the blood from the right ventricle is pumped through the pulmonary arteries to the lungs. There, the blood is oxygenated at the alveoli and returns to the heart through the pulmonary veins. When the heart contracts, the oxygenated blood is pumped throughout the body via a series of thick-walled vessels called arteries. The first and largest artery is called the aorta. The arteries sequentially branch and decrease in size (and are called arterioles) until they end in a network of smaller vessels called capillaries. The capillary beds are located in the interstitial spaces within tissues and release nutrients, immune factors, and oxygen to those tissues. The capillaries connect to a series of vessels called venules, which increase in size to form the veins. The veins join together into larger vessels as they transfer blood back to the heart. The largest veins, the superior and inferior vena cava, return the blood to the right atrium.
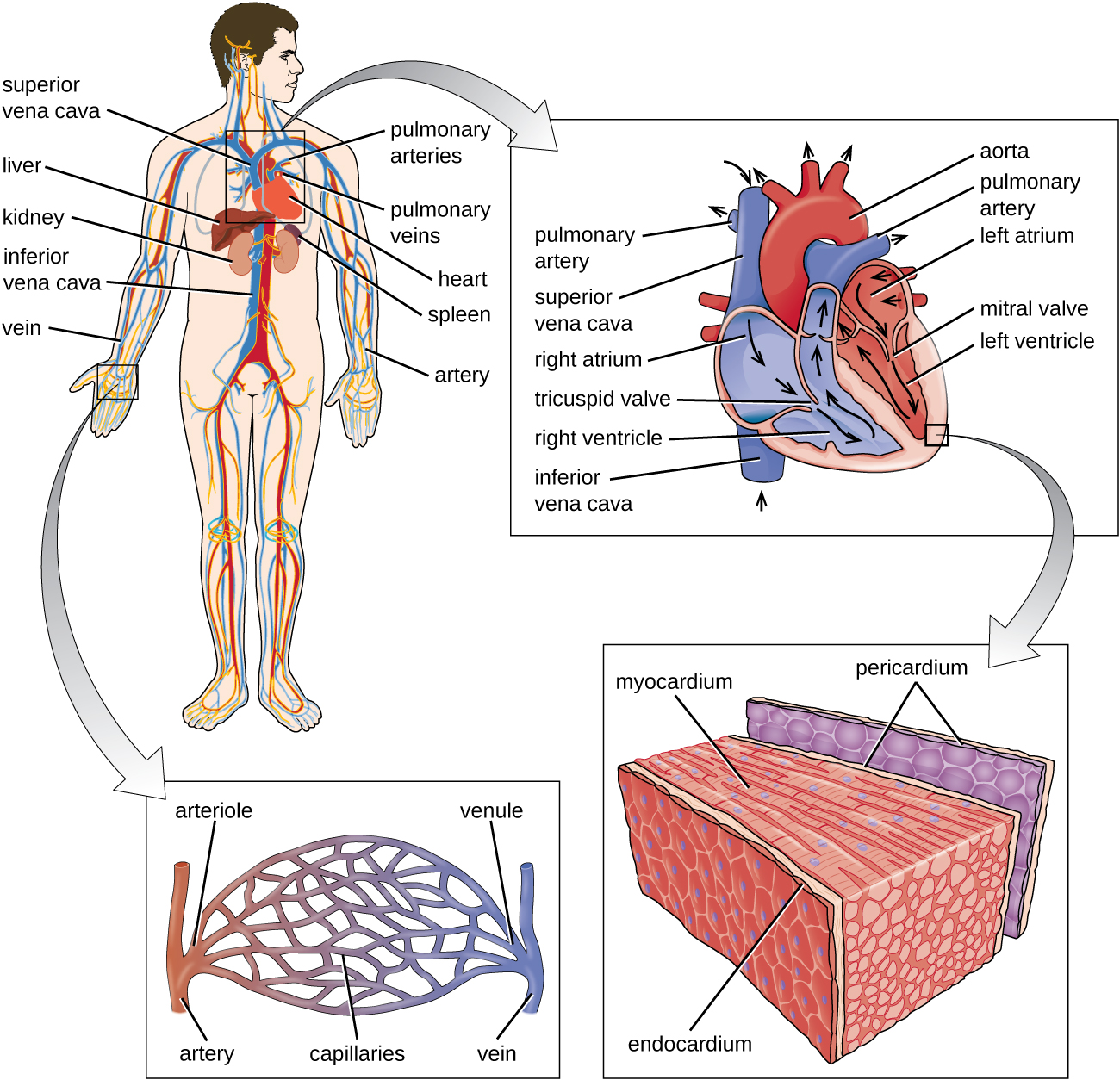
Other organs play important roles in the circulatory system as well. The kidneys filter the blood, removing waste products and eliminating them in the urine. The liver also filters the blood and removes damaged or defective red blood cells. The spleen filters and stores blood, removes damaged red blood cells, and is a reservoir for immune factors. All of these filtering structures serve as sites for entrapment of microorganisms and help maintain an environment free of microorganisms in the blood.
The Lymphatic System
The lymphatic system is also a network of vessels that run throughout the body (Figure \(\PageIndex{12}\)). However, these vessels do not form a full circulating system and are not pressurized by the heart. Rather, the lymphatic system is an open system with the fluid moving in one direction from the extremities toward two drainage points into veins just above the heart. Lymphatic fluids move more slowly than blood because they are not pressurized. Small lymph capillaries interact with blood capillaries in the interstitial spaces in tissues. Fluids from the tissues enter the lymph capillaries and are drained away. These fluids, termed lymph, also contain large numbers of white blood cells.
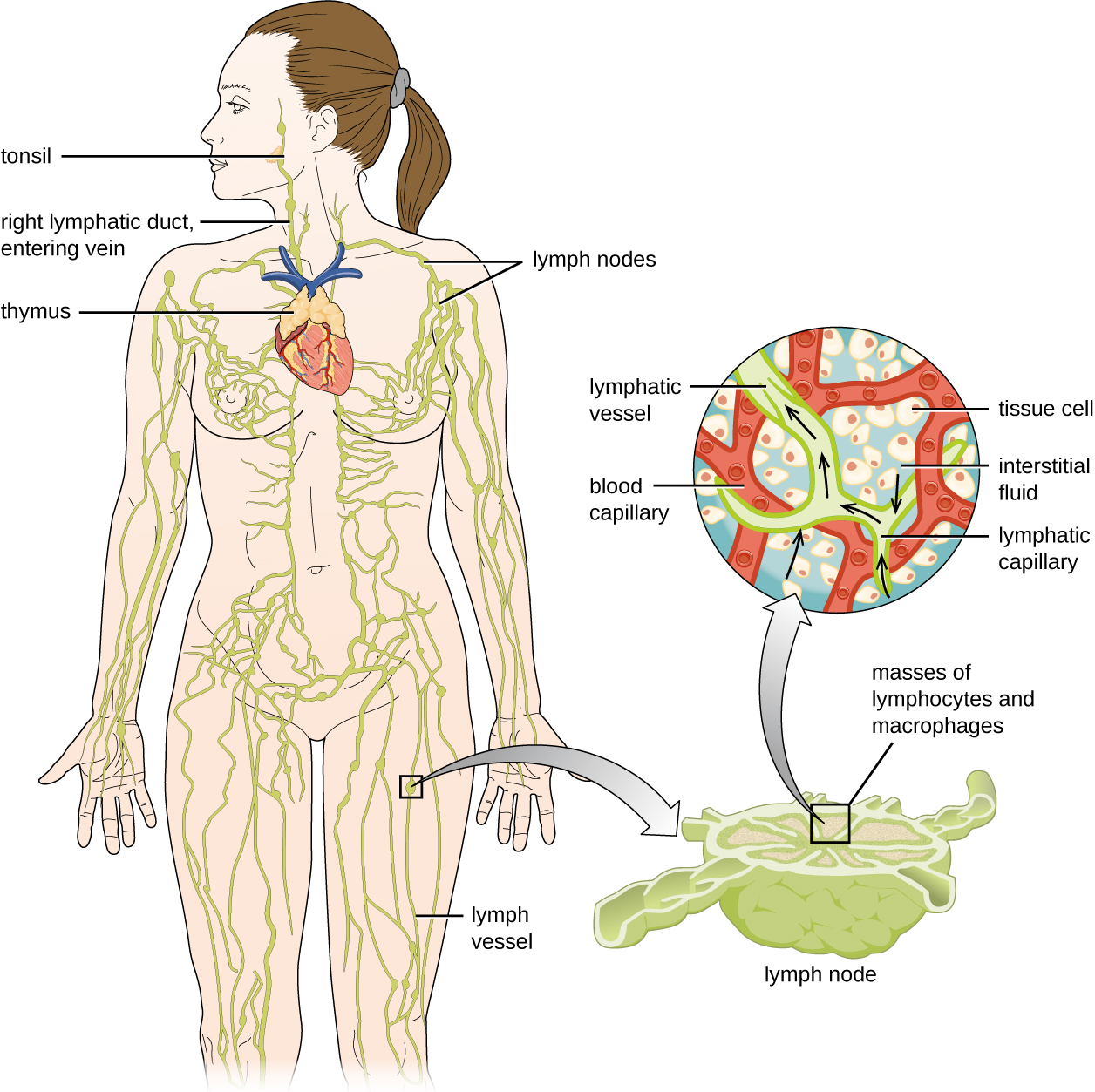
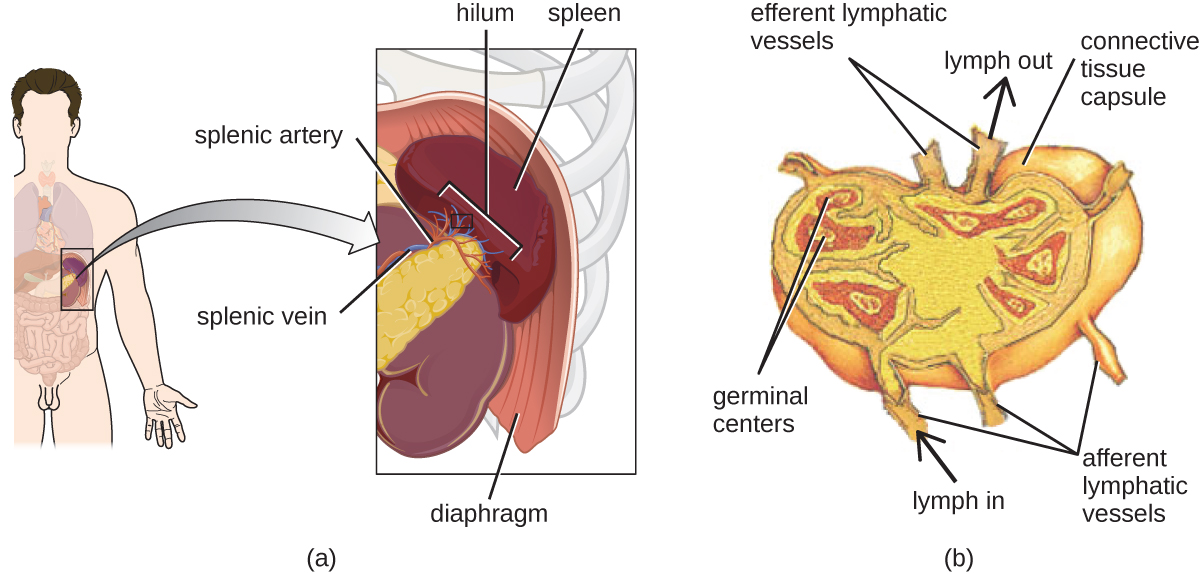
The lymphatic system contains two types of lymphoid tissues. The primary lymphoid tissue includes bone marrow (containing the hematopoietic stem cells) and the thymus. The secondary lymphoid tissues include the spleen, lymph nodes, and several areas of diffuse lymphoid tissues underlying epithelial membranes. The spleen, an encapsulated structure, filters blood and captures pathogens and antigens that pass into it (Figure \(\PageIndex{13}\)). The spleen contains specialized macrophages and dendritic cells that are crucial for antigen presentation, a mechanism critical for activation of T lymphocytes and B lymphocytes in the third line of defense. Lymph nodes are bean-shaped organs situated throughout the body. These structures contain areas called germinal centers that are rich in B and T lymphocytes. The lymph nodes also contain macrophages and dendritic cells for antigen presentation. Lymph from nearby tissues enters the lymph node through afferent lymphatic vessels and encounters these lymphocytes as it passes through; the lymph exits the lymph node through the efferent lymphatic vessels (Figure \(\PageIndex{13}\)).
The lymphatic system filters fluids that have accumulated in tissues before they are returned to the blood. A brief overview of this process is provided at this website.
Exercise \(\PageIndex{5}\)
What is the main function of the lymphatic system?
Key Concepts and Summary
- Plasma contains various proteins that serve as chemical mediators, including acute-phase proteins, complement proteins, and cytokines.
- The complement system involves numerous precursor proteins that circulate in plasma. These proteins become activated in a cascading sequence in the presence of microbes, resulting in the opsonization of pathogens, chemoattraction of leukocytes, induction of inflammation, and cytolysis through the formation of a membrane attack complex (MAC).
- Cytokines are proteins that facilitate various nonspecific responses by innate immune cells, including production of other chemical mediators, cell proliferation, cell death, and differentiation.
- Cytokines play a key role in the inflammatory response, triggering production of inflammation-eliciting mediators such as acute-phase proteins, histamine, leukotrienes, prostaglandins, and bradykinin.
- The formed elements of the blood include red blood cells (erythrocytes), white blood cells (leukocytes), and platelets (thrombocytes). Of these, leukocytes are primarily involved in the immune response.
- All formed elements originate in the bone marrow as stem cells (HSCs) that differentiate through hematopoiesis.
- Granulocytes are leukocytes characterized by a lobed nucleus and granules in the cytoplasm. These include neutrophils (PMNs), eosinophils, and basophils.
- Neutrophils are the leukocytes found in the largest numbers in the bloodstream and they primarily fight bacterial infections.
- Eosinophils target parasitic infections. Eosinophils and basophils are involved in allergic reactions. Both release histamine and other proinflammatory compounds from their granules upon stimulation.
- Mast cells function similarly to basophils but can be found in tissues outside the bloodstream.
- Natural killer (NK) cells are lymphocytes that recognize and kill abnormal or infected cells by releasing proteins that trigger apoptosis.
- Monocytes are large, mononuclear leukocytes that circulate in the bloodstream. They may leave the bloodstream and take up residence in body tissues, where they differentiate and become tissue-specific macrophages and dendritic cells.
- The circulatory system and the lymphatic system provide different but connected transport systems for them proteins and cells to be able to reach (almost) the entire body.
Contributors and Attributions
Nina Parker, (Shenandoah University), Mark Schneegurt (Wichita State University), Anh-Hue Thi Tu (Georgia Southwestern State University), Philip Lister (Central New Mexico Community College), and Brian M. Forster (Saint Joseph’s University) with many contributing authors. Original content via Openstax (CC BY 4.0; Access for free at https://openstax.org/books/microbiology/pages/1-introduction)


