2: Macromolecules
( \newcommand{\kernel}{\mathrm{null}\,}\)
- Understand the structure and bonding properties of carbon and its importance in forming complex molecules essential for life.
- Recognize the role of functional groups in determining the properties and reactivity of organic molecules.
- Identify the major classes of biological macromolecules, including carbohydrates, lipids, proteins, and nucleic acids, and their biological functions.
- Learn how the structure of nucleic acids and proteins is related to their function in cells.
- Explore the importance of carbon-based molecules in metabolic processes, drug development, and cellular functions.
- Carbon Backbone: The central structure formed by carbon atoms that can bond with other atoms to form complex molecules.
- Covalent Bond: A strong bond formed by the sharing of electrons between atoms, especially important in carbon chemistry.
- Functional Groups: Specific atoms or clusters of atoms within molecules that determine their chemical behavior and interactions (e.g., hydroxyl, carboxyl, amino).
- Monosaccharides: Simple sugar molecules that serve as the basic building blocks of carbohydrates (e.g., glucose, fructose).
- Polysaccharides: Complex carbohydrates formed by linking monosaccharides (e.g., starch, glycogen, cellulose).
- Triglycerides: Lipids consisting of one glycerol molecule and three fatty acid chains, serving as an energy storage form.
- Amino Acids: Organic compounds that link together to form proteins, each with distinct side chains (R groups).
- Nucleotides: The building blocks of nucleic acids, consisting of a nitrogenous base, a sugar, and a phosphate group.
- Dehydration Synthesis: A reaction in which water is removed to form larger molecules (e.g., forming a polysaccharide from monosaccharides).
- Hydrolysis: A reaction in which water is added to break down larger molecules (e.g., breaking down a polysaccharide into monosaccharides)
Macromolecules: The Building Blocks of Life
The Importance of Carbon
Carbon is found in all living things! It is the ultimate "Lego" of life because it is lightweight and has four bonding sites, allowing it to form complex and stable molecules. Imagine building with Legos—carbon atoms act like the base pieces that connect to different structures. Because of its ability to form four bonds, carbon creates a strong and flexible backbone for molecules essential to life.
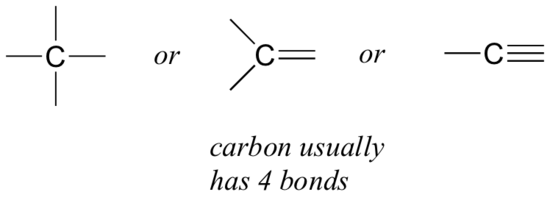
Figure 2.1: Carbon has four bonding sites, allowing for multiple potential complex binding. Image by CC BY-NC-SA 4.0 license
Carbon is a unique element due to its four valence electrons (electrons in its outer shell) and its ability to form four covalent bonds with other atoms (Figure 2.1). The electron configuration of carbon is 1s² 2s² 2p², meaning it needs four more electrons to complete its octet (stable electronic configuration). This results in carbon forming strong, stable covalent bonds with a variety of elements, including hydrogen, oxygen, nitrogen, sulfur, and phosphorus. Below are some advantages that carbon has:
- Versatility in Bonding: Carbon can bond with four different atoms or groups, allowing for an immense diversity of molecular structures.Carbon’s ability to form varied structures leads to a vast array of organic molecules, each with unique chemical and physical properties.
- Formation of Stable Molecules: Carbon forms single, double, or triple bonds, influencing molecular geometry and reactivity. Linear chains provide flexibility, rings offer stability (e.g., aromatic rings), and branching influences solubility and function (Figure 2.2).
- Straight Chains: Carbon atoms can link in a linear fashion, forming molecules like fatty acids and polymers.
- Branched Structures: Some molecules, such as isoprenoids, have branching patterns that alter their chemical properties.
- Rings: Carbon atoms form cyclic structures (e.g., benzene, glucose), which are fundamental in biomolecules like sugars, steroids, and nucleotides.
- Diversity of Organic Compounds: The four bonding sites enable carbon to create small molecules (like methane, CH₄) and massive macromolecules (like DNA and proteins). Shape and structure determine how molecules interact with each other in biological systems.
- Polarity and Solubility: By bonding with different atoms, carbon-containing molecules can be hydrophilic (wate

Figure 2.2: Carbon Structure. This is a diagram showing the four bonding sites of carbon with different atoms and a visual comparison of carbon’s ability to form chains, rings, and branches. Generated via ChatGPT.
Carbon’s structural flexibility and bonding properties make it the foundation of life and biochemistry. Carbon-based molecules are central to metabolism, the set of chemical reactions that sustain life. One key molecule, ATP (adenosine triphosphate), acts as the primary energy currency of the cell, driving essential biological processes. Additionally, carbon chains and rings contribute to the structure of enzyme active sites. These active sites are responsible for binding specific substrates and catalyzing biochemical reactions efficiently, making enzymes indispensable for cellular function.
Many pharmaceutical compounds are carbon-based molecules, including antibiotics, painkillers, and chemotherapy agents. The ability of carbon to form diverse structures allows for the development of a wide range of therapeutic drugs targeting specific biological pathways. Carbon’s structural versatility enables drugs to interact precisely with biological targets, improving their effectiveness while minimizing side effects. Furthermore, modifying the carbon skeleton of drug molecules can enhance their solubility, stability, and bioavailability, optimizing their therapeutic potential.
Overall, carbon’s four bonding sites, chain/ring/branching abilities, and structural diversity make it the backbone of life and biochemistry. Its versatility is essential for biological macromolecules, metabolism, and drug development, reinforcing why carbon-based chemistry is central to science, medicine, and industry.
Functional Groups: The "Equipment" of Molecules
Carbon skeletons can be modified by functional groups, which change the molecule’s properties (Table 2.1). Just like in a video game, where equipping your character with different gear changes their abilities, functional groups affect how molecules fold, bind, and interact with each other. This flexibility allows biological molecules to perform various functions.
Functional groups are specific groups of atoms within molecules that determine their chemical properties and reactivity. These groups influence how molecules interact with each other and participate in biochemical reactions. In organic chemistry and biochemistry, functional groups play a critical role in defining molecular behavior, such as solubility, acidity, polarity, and ability to form hydrogen bonds. Because they dictate how molecules function in biological systems, functional groups are essential in enzymatic reactions, metabolism, and drug interactions.
Common Functional Groups and Their Effects on Molecules
- Hydroxyl Group (-OH):
- The hydroxyl group consists of an oxygen atom bonded to a hydrogen atom (-OH). It is polar due to the oxygen’s high electronegativity, making molecules containing hydroxyl groups hydrophilic (water-soluble). Hydroxyl groups are commonly found in alcohols, carbohydrates, and nucleotides. In biological molecules, hydroxyl groups enable hydrogen bonding, which is essential for protein folding, enzyme-substrate interactions, and DNA stability.
- Carboxyl Group (-COOH):
- The carboxyl group is a combination of a carbonyl (-C=O) and hydroxyl (-OH) group attached to the same carbon. It is found in amino acids, fatty acids, and carboxylic acids. Carboxyl groups are acidic, meaning they can donate a hydrogen ion (H⁺) in solution, influencing pH levels in biological systems. This property is crucial in protein structure and enzyme function, as pH changes can alter molecular shape and reactivity.
- Amino Group (-NH₂):
- The amino group consists of a nitrogen atom bonded to two hydrogen atoms (-NH₂). It is basic, meaning it can accept a proton (H⁺) and become positively charged (-NH₃⁺) in physiological conditions. Amino groups are a fundamental part of amino acids, which form proteins. They contribute to hydrogen bonding, protein folding, and enzyme catalysis, making them essential for biological activity.
- Phosphate Group (-PO₄³⁻):
- The phosphate group consists of a phosphorus atom bonded to four oxygen atoms, often carrying a negative charge. Phosphate groups are crucial in energy transfer and storage in biological systems, as seen in ATP (adenosine triphosphate), the main energy carrier of cells. They also play a significant role in DNA and RNA structure, cell signaling, and enzyme regulation. The presence of a phosphate group increases molecule solubility and reactivity, which is why it is widely used in metabolism.
- Carbonyl Group (-C=O):
- The carbonyl group contains a carbon atom double-bonded to an oxygen atom. It is found in ketones and aldehydes, which are present in sugars, steroids, and metabolic intermediates. Carbonyl groups influence polarity, reactivity, and hydrogen bonding, making them key in metabolic pathways and enzyme function.
- Sulfhydryl Group (-SH):
- The sulfhydryl group consists of a sulfur atom bonded to a hydrogen atom. It is found in cysteine, an amino acid that helps form disulfide bonds (-S-S-), which are crucial for protein stability and structure. Disulfide bridges are essential in enzyme function and cellular signaling.
Functional groups are the active sites of biological molecules, determining how they interact with enzymes, receptors, and other biomolecules. Enzymes recognize specific functional groups in substrates, allowing them to catalyze chemical reactions efficiently. For example, enzymes that break down carbohydrates recognize hydroxyl and carbonyl groups, while those involved in protein digestion target amino and carboxyl groups. In drug development, functional groups influence drug solubility, absorption, and target interaction. Many drugs mimic or modify natural functional groups to enhance bioavailability and efficacy. For instance, aspirin contains a carboxyl group that interacts with enzymes to reduce inflammation, and many antibiotics contain hydroxyl and amine groups that disrupt bacterial processes.
Overall, Functional groups are the foundation of molecular interactions in biology and chemistry. They dictate the properties of biomolecules, determine how enzymes function, and play a vital role in drug action. Understanding functional groups is essential for biochemistry, medicine, and pharmaceuticals, as they influence the structure, stability, and reactivity of life’s most important molecules.

Table 2.1: This is a chart displaying common functional groups (hydroxyl, carboxyl, amino, phosphate, etc.) and their effects on molecules.
Functions of Carbohydrates
Carbohydrates provide energy and structural support to cells (Table 2.2). They are made of monomers called monosaccharides (such as glucose, fructose, and galactose). When these monomers link together, they form polysaccharides (such as starch, glycogen, cellulose, and peptidoglycan) (Figure 2.3). Carbohydrates are essential biological molecules that serve as a primary source of energy, play a role in structural support, and contribute to cell signaling and molecular recognition. They are composed of carbon (C), hydrogen (H), and oxygen (O) in a typical ratio of 1:2:1. Carbohydrates are classified based on their complexity into monosaccharides, disaccharides, and polysaccharides, each serving different functions in biological systems.
Monosaccharides → Disaccharides → Polysaccharides
- Monosaccharides (Simple Sugars)
- Basic units of carbohydrates consist of a single sugar molecule.
- Examples: Glucose, Fructose, Galactose
- Disaccharides (Two Sugar Units)
- Formed by the condensation of two monosaccharides through dehydration synthesis.
- Examples: Sucrose (Glucose + Fructose), Lactose (Glucose + Galactose), Maltose (Glucose + Glucose)
- Polysaccharides (Complex Carbohydrates)
- Long chains of monosaccharides linked together, serving as energy storage or structural components.
- Examples: Starch, Glycogen, Cellulose, Chitin, Peptidoglycan
Comparison of Starch, Glycogen, and Cellulose
| Property | Starch (Plants) | Glycogen (Animals) | Cellulose (Plants) |
|---|---|---|---|
| Structure | Linear & branched (Amylose & Amylopectin) | Highly branched | Linear, rigid |
| Function | Energy storage in plants | Energy storage in animals | Structural support in plants |
| Linkages | α(1→4) and α(1→6) | α(1→4) and α(1→6) | β(1→4) |
| Digestibility | Easily digested | Easily digested | Humans cannot digest it |
Table 2.2: Comparison between different polysaccharides
Energy Storage Functions of Carbohydrates
1. Starch (Plants’ Energy Reserve)
Starch is the primary storage carbohydrate in plants, found in seeds, tubers, and roots (e.g., potatoes, rice). A baked potato or French fries are rich in starch, which the body digests into glucose for energy. Rice and bread are also common starch sources. Starch can also consists of two forms:
- Amylose: Linear chains of glucose molecules linked by α(1→4) bonds.
- Amylopectin: Branched structure with α(1→6) linkages, making it easier to break down for quick energy.
2. Glycogen (Animals’ Energy Reserve)
Glycogen is the primary carbohydrate storage molecule in animals, stored mainly in the liver and muscles. It has a highly branched structure, allowing rapid hydrolysis for quick energy release during exercise or fasting. Beef liver contains glycogen, which provides quick energy when needed. However, most glycogen in animals breaks down after slaughter, so it's not a major dietary source.
Carbohydrates in Cell Structure
1. Cellulose (Plants & Algae)
Cellulose is the most abundant organic polymer on Earth, forming the structural component of plant cell walls. It consists of β(1→4) glucose linkages, which create long, unbranched fibers that provide rigidity and strength to plants. Some structural carbohydrates, like cellulose and chitin, can also serve as an energy source for certain organisms. For example, some bacteria, including those in our gut, and herbivores have specialized enzymes to break down cellulose, allowing them to digest plants. Also, celery and kale have tough, fibrous textures because of cellulose, which provides structure to plant cell walls. Even though we can’t digest cellulose, it acts as dietary fiber, helping digestion. Humans cannot digest cellulose because we lack the enzyme cellulase, which breaks β(1→4) glycosidic bonds. However, some bacteria in our gut microbiome can partially break down cellulose, producing short-chain fatty acids that provide some energy. These gut microbes help with digestion, gut health, and immune function
2. Chitin (Insects & Fungi)
Chitin is a modified carbohydrate forming the exoskeleton of insects, crustaceans, and the cell walls of fungi. It consists of N-acetylglucosamine (a derivative of glucose), giving it extra strength and durability. Mushrooms like shiitake and portobello contain chitin in their cell walls, giving them a slightly chewy texture. Also, the crunchy shell of shrimp or crabs is made of chitin.
3. Peptidoglycan (Bacteria)
Peptidoglycan is a structural carbohydrate found in bacterial cell walls, consisting of sugar chains cross-linked by amino acids. This structure gives bacteria shape and protection from environmental stress. Some antibiotics, like penicillin, target peptidoglycan to weaken bacterial cell walls and kill bacteria.
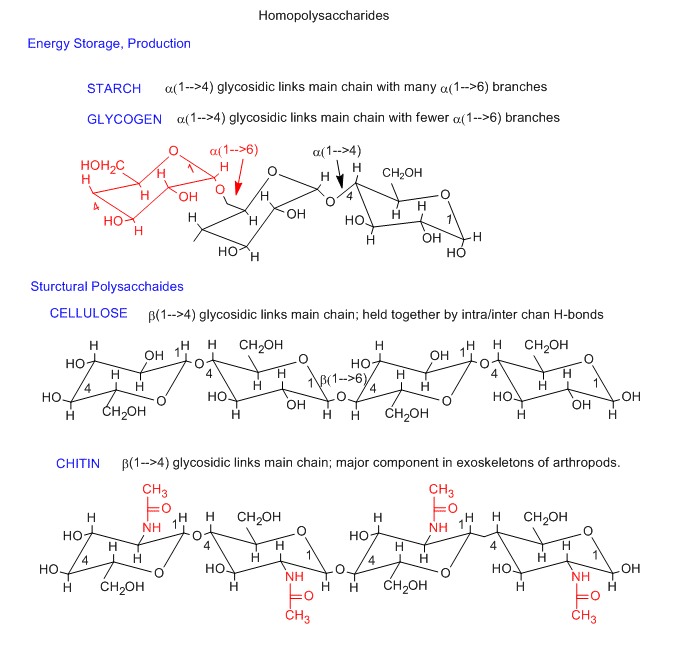
Figure 2.3: Image of starch, glycogen, cellulose, and Chitin. Image from CC BY-NC 3.0 license
Hydrolysis vs. Dehydration Synthesis (Figure 2.4)
-
Dehydration Synthesis (Condensation Reaction)
- Monosaccharides (simple sugars) join together to form disaccharides or polysaccharides by removing water (H₂O), or releasing a water molecule to form a polysaccharide.
- Example: Glucose + Fructose → Sucrose + H₂O
-
Hydrolysis (Breaking Down Carbohydrates)
- Polysaccharides are broken down into monosaccharides by adding water (H₂O), reversing dehydration synthesis. Think of cotton candy—when it touches your tongue, it dissolves quickly due to hydrolysis!
- Example: Sucrose + H₂O → Glucose + Fructose
Figure 2.4: Polymer formation. Dehydration reactions join monomers. Hydrolysis splits polymers.
Carbohydrates are critical in biochemistry because they provide energy, structural integrity, and cellular communication. Many enzymes specialize in metabolizing carbohydrates, such as amylase (breaks down starch), lactase (breaks down lactose), and cellulase (not found in humans but present in some bacteria and fungi). In drug development, carbohydrates play a role in vaccine design, targeting cancer cells, and antiviral therapies. For example, heparin (a polysaccharide) is used as a blood thinner, and some antibiotics (e.g., streptomycin) contain sugar molecules that enhance their function.
Overall, Carbohydrates are essential macromolecules involved in energy storage, structural support, and biochemical signaling. Their diversity in structure—ranging from simple sugars to complex polysaccharides—allows them to perform multiple biological roles. Understanding their functions is crucial in biochemistry, nutrition, medicine, and biotechnology.
Lipids: Structure, Function, and Importance
Lipids are an essential class of biological macromolecules that play crucial roles in energy storage, cellular structure, hormone production, and protection. Unlike proteins and carbohydrates, lipids are hydrophobic (water-repelling), making them ideal for forming barriers like cell membranes. There are several types of lipids, including triglycerides, phospholipids, steroids, and waxes, each with unique functions and biological significance.
1. Triglycerides: The Body’s Energy Reserves
Triglycerides are composed of one glycerol molecule and three fatty acid chains (Figure 2.5). These fatty acids can be either saturated (solid at room temperature) or unsaturated (liquid at room temperature). Triglycerides serve as the primary energy storage molecules in animals and plants. When the body requires energy, specialized enzymes called lipases break down triglycerides into glycerol and fatty acids, which can then be converted into ATP through cellular respiration.
Triglycerides are primarily stored in adipose tissue—the body’s fat deposits found under the skin (subcutaneous fat) and around organs (visceral fat). In animals, they are stored in white adipose tissue (WAT) for long-term energy storage, while brown adipose tissue (BAT) is responsible for generating heat, particularly in newborns and hibernating animals. Small amounts of triglycerides are also stored in muscle cells, where they can be quickly broken down for energy during exercise.
Fatty acids in triglycerides can be classified as saturated or unsaturated, affecting their physical properties and health effects (Table 2.3).
- Saturated fatty acids have no double bonds in their hydrocarbon chains, making them solid at room temperature. They are commonly found in animal fats (butter, lard, red meat) and tropical oils (coconut oil, palm oil). High consumption of saturated fats is linked to an increase in LDL (low-density lipoprotein) cholesterol, which can contribute to heart disease.
- Unsaturated fatty acids contain one or more double bonds, making them liquid at room temperature. They are found in olive oil, avocados, nuts, and fatty fish (salmon, tuna, mackerel). These fats are beneficial because they increase HDL (high-density lipoprotein) cholesterol, which helps remove excess cholesterol from the blood.
Cholesterol is transported through the bloodstream by lipoproteins, mainly LDL ("bad" cholesterol) and HDL ("good" cholesterol). LDL can deposit cholesterol in artery walls, leading to plaque buildup (atherosclerosis), high blood pressure, and increased risk of heart attack or stroke. HDL, on the other hand, helps remove excess cholesterol and transport it to the liver for excretion, reducing cardiovascular risk.

Figure 2.5: Image of Triglyceride.
| Type | Structure | Sources | Effects on Health |
|---|---|---|---|
| Saturated Fatty Acids | No double bonds (solid at room temp) | Butter, red meat, cheese, coconut oil | Raises LDL ("bad" cholesterol) |
| Unsaturated Fatty Acids | One or more double bonds (liquid at room temp) | Olive oil, nuts, avocados, fish | Raises HDL ("good" cholesterol), lowers LDL |
Table 2.3: Saturated vs. Unsaturated Fatty Acids
2. Phospholipids: The Cell Membrane Barrier
Phospholipids are crucial structural components of cell membranes, forming the phospholipid bilayer that separates the inside of the cell from its external environment (Figure 2.5). A phospholipid molecule consists of a hydrophilic (water-loving) phosphate head and two hydrophobic (water-repelling) fatty acid tails. When exposed to water, phospholipids arrange themselves into a bilayer, with the hydrophobic tails facing inward and the hydrophilic heads facing outward, creating a selectively permeable membrane that regulates the movement of substances in and out of cells.
This bilayer acts as the “skin” of our cells, providing structure while also allowing communication, transport of molecules, and protection. Proteins embedded within the membrane function as receptors, channels, and transporters, enabling essential nutrients to enter and waste products to exit. Without phospholipids, cells would not be able to maintain their internal environment, ultimately leading to cell death.

Figure 2.5: Illustration of the Human cell membrane structure from https://www.freepik.com/author/brgfx
3. Steroids: Cholesterol and Hormones
Steroids are lipids with a distinctive four-ring carbon structure. Cholesterol is the most well-known steroid, serving as the precursor for important hormones, including testosterone, estrogen, cortisol, and vitamin D (Figure 2.6). While cholesterol is essential for various bodily functions, excessive levels of LDL cholesterol can contribute to plaque buildup in arteries, increasing the risk of hypertension, heart attacks, and strokes.
When LDL cholesterol accumulates in blood vessels, it forms plaques that narrow arteries, restricting blood flow. This leads to high blood pressure because the heart must work harder to pump blood through narrowed pathways. If a plaque ruptures, it can cause a blood clot, potentially leading to a heart attack (if in coronary arteries) or a stroke (if in the brain).

Figure 2.6: Image of Cholesterol leading to other precursors. Generated via ChatGPT
Despite its risks, cholesterol is vital for:
- Vitamin D production, which helps in calcium absorption and bone health.
- Sex hormone synthesis, including testosterone and estrogen, which regulate reproduction and secondary sexual characteristics.
- Maintaining cell membrane fluidity, allowing cells to function properly.
Some steroid hormones, such as testosterone and estrogen creams, can be absorbed through the skin and enter the bloodstream, making transdermal hormone therapy possible. Steroid hormones also regulate gene expression, turning specific genes on or off to control various biological functions. Anabolic steroids, synthetic derivatives of testosterone, are sometimes used by athletes to increase muscle mass, strength, and endurance. However, their misuse can lead to severe health issues, including heart disease, liver damage, mood disorders (e.g., "roid rage"), and infertility.
4. Waxes: Nature’s Waterproofing Agent
Waxes are composed of long-chain fatty acids linked to alcohols or hydrocarbons, making them highly hydrophobic and ideal for waterproofing surfaces in plants and animals. Wax can be found in:
- Humans: Earwax (cerumen) is produced in the ear canal to trap dust, dirt, and microorganisms, preventing infections. The skin and hair also have a thin layer of sebum, a waxy secretion that helps retain moisture and protect against pathogens.
- Plants: The waxy cuticle on leaves and fruits prevents water loss and protects against environmental damage.
- Animals: Bees produce beeswax, used to build honeycombs, while some birds secrete a waxy coating to waterproof their feathers.
Overall, Lipids are essential to life, playing diverse roles in energy storage, cellular structure, hormone signaling, and protection. While some fats, such as unsaturated fats and phospholipids, contribute to good health, excessive saturated fats and LDL cholesterol can lead to severe health problems. Understanding lipids is critical in fields like biochemistry, medicine, nutrition, and pharmacology, as they influence everything from cell function to disease prevention and drug development.
Proteins: Structure, Function, and Importance
Proteins are made of 20 different amino acids (Figure 2.7). These amino acids act like letters in a language—just as you can form countless sentences using a limited alphabet, proteins can take on millions of forms with different functions. Proteins are among the most essential macromolecules in living organisms, responsible for structural support, enzymatic activity, transport, signaling, and defense mechanisms. These molecules are made up of amino acids, which are linked together in specific sequences to form unique structures that determine their function. The shape of a protein is critical for its role in biological systems, and understanding protein structure is fundamental in biochemistry, medicine, and drug development.
All proteins are made up of amino acids, which share a common structure shown in Figure 2.8.
- A central carbon (α-carbon)
- A hydrogen atom (H)
- An amino group (-NH₂)
- A carboxyl group (-COOH)
- A variable side chain (R-group) that determines the properties of the amino acid.
- The R-group (side chain) varies between different amino acids, giving each one unique chemical properties. Some are hydrophilic (water-attracting), hydrophobic (water-repelling), acidic, or basic, influencing how they interact and fold into functional proteins.

Figure 2.7: Types of amino acids: There are 21 common amino acids commonly found in proteins, each with a different R group (variant group) that determines its chemical nature. The 21st amino acid, not shown here, is selenocysteine, with an R group of -CH2-SeH. Shared under a CC BY-SA license

Figure 2.8: Image of an Amino Acid.
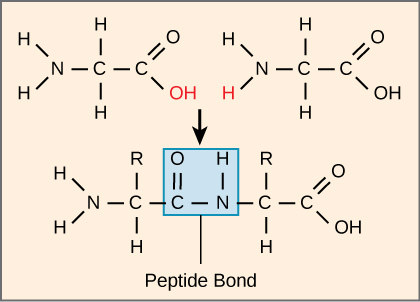
Figure 2.9: Peptide bond formation: Peptide bond formation is a dehydration synthesis reaction. The carboxyl group of one amino acid is linked to the amino group of the incoming amino acid. In the process, a molecule of water is released. Shared under a CC BY-SA license
Proteins fold into complex three-dimensional structures, which are categorized into four levels of organization.
- The primary structure of a protein is the linear sequence of amino acids linked together by peptide bonds (Figure 2.9). This sequence is encoded by DNA and determines the protein’s final shape and function. Even a single mutation in this sequence can alter the protein's activity, sometimes leading to diseases like sickle cell anemia.
- The secondary structure forms when the amino acid chain folds into patterns, stabilized by hydrogen bonds between nearby amino and carboxyl groups. The most common patterns are:
- Alpha-helix (α-helix) – A spiral shape stabilized by hydrogen bonds. Found in structural proteins like keratin (hair, nails).
- Beta-pleated sheet (β-sheet) – A folded sheet-like structure. Found in strong proteins like silk.
- The tertiary structure refers to the overall three-dimensional folding of a protein due to interactions between R-groups. This structure determines the function of enzymes, antibodies, and transport proteins. For example, the enzyme lysozyme folds in a way that allows it to break down bacterial cell walls. These interactions include:
- Hydrogen bonding (helps stabilize the structure)
- Ionic bonds (between charged R-groups)
- Hydrophobic interactions (hydrophobic side chains group together inside the protein, away from water)
- Disulfide bridges (strong covalent bonds between sulfur-containing amino acids like cysteine)
- Some proteins consist of multiple polypeptide chains (subunits) that join together to form a functional unit. This is called the quaternary structure. Examples include:
- Hemoglobin (a protein in red blood cells that carries oxygen, composed of four subunits).
- Collagen (a structural protein in skin and connective tissues, made of three intertwined chains).
Proteins must fold correctly to function properly. If a protein misfolds, it can lead to diseases such as Alzheimer’s, Parkinson’s, and cystic fibrosis, where misfolded proteins aggregate into harmful plaques or fibers. In drug development, understanding protein structure is crucial. Scientists use X-ray crystallography and cryo-electron microscopy to determine protein structures and design drugs that fit into their active sites, like a lock and key mechanism. Many drugs work by binding to specific proteins to either activate or inhibit their function. For example:
- Antibiotics target bacterial enzymes, preventing them from functioning.
- Cancer drugs block proteins that promote uncontrolled cell growth.
- Insulin (a hormone protein) regulates blood sugar levels in diabetes treatment.
Overall, proteins are vital molecules in all living organisms, involved in nearly every biological process. Their structure determines their function, and even slight changes in folding can have profound effects on health. Understanding protein structure is essential for biochemistry, medicine, and drug design, helping researchers develop new treatments for diseases. Through techniques like protein engineering and drug targeting, scientists continue to explore the incredible potential of proteins in biotechnology and healthcare.
Nucleic Acids: Structure, Function, and Biological Importance
Nucleic acids are biological macromolecules that store and transmit genetic information and are essential for cellular functions. The two main types are DNA (deoxyribonucleic acid) and RNA (ribonucleic acid). These molecules are composed of nucleotides, which serve as the building blocks of nucleic acids. In addition to genetic storage, nucleotides play vital roles in energy transfer (ATP), catalysis (ribozymes), and molecular recognition (aptamers).
Each nucleotide consists of three main components as shown in Figure 2.10.
- A five-carbon sugar (pentose):
- Deoxyribose in DNA
- Ribose in RNA
- A phosphate group, which links nucleotides together in a chain via phosphodiester bonds.
- A nitrogenous base, which determines the identity of the nucleotide:
- Purines (double-ring structures): Adenine (A), Guanine (G)
- Pyrimidines (single-ring structures): Cytosine (C), Thymine (T) in DNA, Uracil (U) in RNA
Table 2.4 describes the differences between DNA and RNA. The sugar-phosphate backbone forms the structural framework of DNA and RNA, while the nitrogenous bases pair together to encode genetic information. The absence of the 2'-OH group in deoxyribose makes DNA less reactive and more resistant to hydrolysis. This stability is crucial for long-term genetic storage, as DNA must remain intact over generations. RNA, with its extra hydroxyl (-OH) group at the 2' position, is more chemically reactive and can catalyze biochemical reactions (ribozymes). This makes RNA ideal for transient roles like messenger RNA (mRNA) and transfer RNA (tRNA). DNA also exists as a double-helix, where two strands wind around each other in an antiparallel fashion (5’→3’ direction of one strand runs opposite to the other) (Figure 2.11). The 3D structure of DNA is crucial for processes like replication, transcription, and epigenetic modifications, influencing gene expression and cellular function.The helical structure is stabilized by the hydrogen bonds between complementary base pairs.

Figure 2.10: Image of a Nucleic Acid.
| Feature | DNA (Deoxyribonucleic Acid) | RNA (Ribonucleic Acid) |
|---|---|---|
| Sugar | Deoxyribose (lacks 2'-OH) | Ribose (has 2'-OH) |
| Strands | Double-stranded (helix) | Single-stranded |
| Bases | A, T, C, G | A, U, C, G |
| Stability | More stable, less reactive | More reactive (due to 2'-OH) |
| Function | Long-term genetic storage | Involved in protein synthesis, gene regulation, catalysis |
Table 2.4: Differences between DNA and RNA.

Figure 2.11: DNA built from a 5' to 3' direction
In DNA and RNA, nitrogenous bases pair through hydrogen bonding following Chargaff’s base-pairing rules:
- Adenine (A) pairs with Thymine (T) in DNA and Uracil (U) in RNA → 2 hydrogen bonds
- Cytosine (C) pairs with Guanine (G) → 3 hydrogen bonds
The additional hydrogen bond in C-G pairs makes them stronger than A-T pairs, which is critical in maintaining DNA stability. Organisms in high-temperature environments (e.g., thermophilic bacteria) have a higher proportion of C-G base pairs, as they prevent DNA strands from denaturing. At the ends of eukaryotic chromosomes, DNA sequences called telomeres serve as protective caps, preventing loss of essential genetic information during replication. Telomeres consist of repetitive nucleotide sequences (e.g., TTAGGG in humans) and do not code for proteins. During DNA replication, the lagging strand cannot be fully copied, leading to progressive shortening of chromosomes. Telomerase, an enzyme active in stem cells and cancer cells, extends telomeres by adding repeated sequences, counteracting this loss. Without telomerase, telomeres gradually shorten, leading to cell aging (senescence) and eventual cell death. The repetitive G-rich sequences in telomeres can form G-quadruplex structures, stabilized by hydrogen bonds. These structures help protect chromosome ends from degradation and fusion with neighboring chromosomes.
Nucleotides also serves other functions as well. For example, ATP, which is a nucleotide derivative that acts as the primary energy currency of the cell. It consists of Adenine base, Ribose sugar, and Three phosphate groups. When ATP is hydrolyzed into ADP (Adenosine Diphosphate) and inorganic phosphate (Pi), it releases energy used for cellular functions such as muscle contraction, active transport, and enzyme activity.
- Reaction: ATP → ADP + Pi + Energy
Other Functions of Nucleotides
- rRNA (Ribosomal RNA): A structural and catalytic component of ribosomes, essential for protein synthesis.
- tRNA (Transfer RNA): Carries amino acids to ribosomes during translation.
- Aptamers: Short, single-stranded DNA or RNA sequences that bind specifically to target molecules (used in diagnostics and therapeutics).
- Coenzymes (e.g., NAD+, FAD, Coenzyme A): Derived from nucleotides, these molecules assist in metabolic reactions like cellular respiration.
Overall, Nucleic acids are fundamental to life, storing genetic information, regulating gene expression, and facilitating biological reactions. DNA's stability and structure allow for long-term genetic storage, while RNA's versatility enables various cellular functions. Understanding these molecules is essential in biochemistry, medicine, and drug development, as it aids in advancements like gene therapy, RNA-based vaccines, and targeted cancer treatments.
- Understand Carbon’s Versatility: Recognize how carbon’s ability to form various bonding patterns (straight chains, branched chains, rings) leads to a wide variety of organic molecules.
- Analyze the Role of Functional Groups: Understand the role functional groups play in modifying the behavior of molecules in biological systems.
- Link Structure to Function in Biological Molecules: See how the structure of molecules such as carbohydrates, lipids, proteins, and nucleic acids influences their function in cells.
- Explore the Relationship Between Structure and Disease: Learn how misfolded proteins or genetic mutations related to carbon-based molecules contribute to diseases like Alzheimer's, cancer, and sickle cell anemia.
- Understand the Biochemical Role of ATP: Identify ATP as the energy currency of cells and explain how it’s used in biochemical reactions.
- ATP (Adenosine Triphosphate): A nucleotide that serves as the primary energy carrier in cells, used in processes like muscle contraction and active transport.
- Polysaccharides: Large carbohydrate molecules made from many monosaccharides (e.g., starch, glycogen, cellulose) that store energy or provide structural support.
- Phospholipids: Molecules that form the lipid bilayer of cell membranes; consist of a hydrophilic head and hydrophobic tails, enabling selective permeability.
- Steroids: Lipids with a four-ring carbon structure, like cholesterol, which are precursors for hormones and maintain membrane fluidity.
- Protein Structure Levels: The four levels of protein structure (primary, secondary, tertiary, and quaternary) determine the shape and function of proteins.
- Denaturation: The process by which proteins lose their shape and function, often due to temperature or pH changes.
- Nucleotides: The building blocks of nucleic acids, containing a sugar, phosphate group, and nitrogenous base, also involved in energy transfer (e.g., ATP).
- How does the misfolding of proteins contribute to diseases like Alzheimer's or cancer?
- Discuss how carbon’s versatile bonding properties relate to the development of various therapeutic drugs (e.g., antibiotics, chemotherapy agents).
- Present a case where the disruption of protein folding or the failure of carbon-based molecules in metabolism leads to disease.
- Functional Group Impact: Think about how modifying the functional groups on a carbon skeleton alters molecular behavior (e.g., solubility, reactivity, or enzyme binding).
- Carbohydrate Function: Review how carbohydrates serve as energy sources and structural materials (e.g., cellulose for plants, glycogen for animals).
- Lipids in Energy Storage and Cell Structure: Understand how lipids function in long-term energy storage (triglycerides), cellular membranes (phospholipids), and signaling (steroids).
- Protein Folding and Structure: Reflect on the four levels of protein structure (primary to quaternary) and how they determine protein function in enzymes, transporters, and structural components.
- Nucleic Acid Functions: Revisit the importance of DNA and RNA in genetic information storage and transfer, and how nucleotides serve additional roles in energy metabolism and cellular function.
- Enzyme and Substrate Interaction: Consider how enzymes recognize specific molecules through functional groups, affecting biochemical pathways and drug development.

