1.22: Fermentation
- Page ID
- 79455
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Explain what fermentation is and why it is important for microorganisms.
- Give examples of types of fermentation products, including fermentation products used by humans.
- Tell how fermentation tests can be useful in identification and characterization of bacterial species.
- Describe how the fermentation test works including the functions of phenol red and Durham tubes.
- Tell that fermentation can utilize different carbohydrates resulting in different fermentation reactions.
- Successfully conduct and interpret fermentation tests.
Fermentation is a Metabolic Process
Fermentation is a metabolic process that some microorganisms use to break down glucose and other sugars when O2 is not available or could not be used by the microorganism. Fermentation is a way bacteria can produce ATP to meet their energy needs (although fermentation produces significantly less ATP than aerobic respiration or anaerobic respiration). Fermentation includes the metabolic pathway glycolysis (where a single molecule of glucose is broken down into 2 molecules of pyruvate), as well as additional fermentation reactions that produce a variety of end products (acids, alcohols, gases). The end products are characteristic of individual bacterial species. Fermentation is also possible from non-sugar molecules. Even unusual compounds like aromatics (benzoate), glycerol (sugar-alcohol), and acetylene (hydrocarbons) may be fermented by some bacterial species!
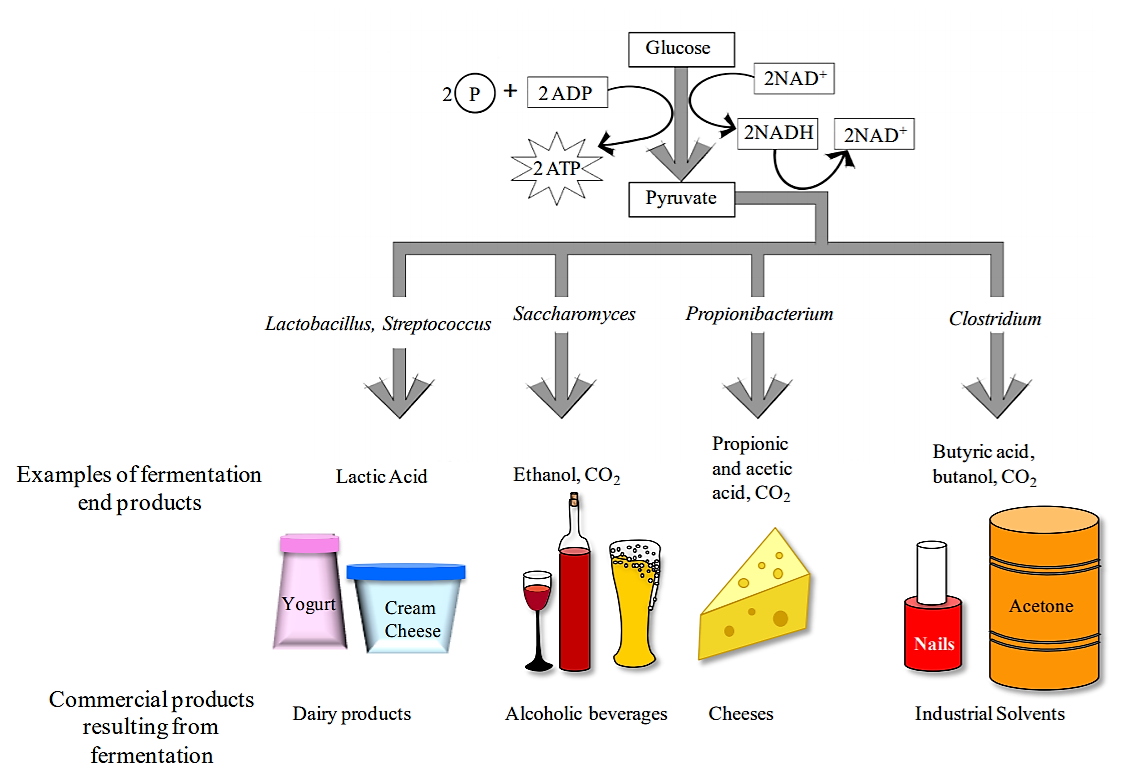
Figure 1: Carbohydrate fermentation. Fermentation is a microbial process utilized to produce a variety of products used by humans including dairy products (yogurt and cream cheese), alcoholic beverages, cheeses, and industrial solvents. Fermentation is a metabolic process the begins with glycolysis to make a small amount of ATP and pyruvate. Pyruvate then undergoes additional fermentation reactions resulting in fermentation byproducts, including those important to humans.
Note that fermentation is mainly a mechanism used by cells to regenerate NAD+ so that NAD+ is available for glycolysis to continue when cellular respiration is not occurring. Fermentation also tends to produce waste products that can accumulate in the extracellular environment. By contrast, the waste left over after ATP production by aerobic respiration are limited to CO2 and H2O. There can be numerous end products from fermentation, many of which is useful for us humans, but not necessarily the microbes. We use many fermentation products--as diverse as antibiotics, alcohols, and a variety of foods. Microbes such as yeast (e.g. Saccharomyces cerevisiae) and bacteria are genetically engineered to produce valuable fermentation products.
Much of the original energy in the substrate remains within the chemical bonds of organic end products such as lactic acid or ethanol. For example, ethanol has so much stored energy it can be used in gasoline solutions to be combusted/burned to release that energy stored in its chemical bonds!
Fermentation of a Variety of Carbohydrates
Bacteria, depending on the species, can ferment different carbohydrates. Although glycolysis (the pathway leading to fermentation) begins with glucose, some bacteria have the enzymes needed for additional chemical reactions to convert other monosaccharides (e.g. fructose and mannose) as well as disaccharides (e.g. lactose and sucrose) so they can enter the glycolysis pathway. These bacteria therefore require the genes in their DNA that code for enzymes capable of converting these sugars into molecules that can enter glycolysis. For example, the enzyme beta-galactosidase is necessary to break down lactose. This enzyme is coded in the DNA of microbes that can metabolize lactose so they can produce this enzyme.
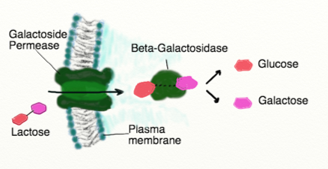
Figure 2: Lactose on the outside of a bacterial cell can be brought into the cell if the cell has the enzyme permease to transport it. Lactose metabolism also requires beta-galactosidase to break down the disaccharide lactose into monosaccharides.
Therefore bacteria can be differentiated both based on their ability to ferment various carbohydrates, as well as the end products that result from the fermentation process. As a result, examining the ability of bacterial species to ferment a variety of carbohydrates is an approach used to characterize bacterial species and is useful for species identification.
Fermentation Test
Some bacteria will produce gases when fermenting a carbohydrate. To detect these gases, a Durham tube is used. This is a small inverted glass tube that is placed within the larger glass tube containing the fermentation medium. If gases (typically CO2) are produced during the fermentation process, a bubble will form at the top of the Durham tube. If you see a bubble in the Durham tube, this means fermentation occurred and gas was produced during fermentation.

Figure 3: Diagram showing the fermentation test setup with a test tube containing medium and a tiny tube inside of the medium that is upside down to capture gases produced during fermentation. This tiny upside down tube is called a Durham tube. If gas is produced during fermentation, there will be a space in the top of the Durham tube that does not have any medium in it since gas has displaced the medium at the top of the tube forming a bubble.
The medium used to test carbohydrate fermentation is a nutrient broth that contains a fermentable carbohydrate (usually a monosaccharide or a disaccharide), peptone (amino acids) as well as a pH indicator. The pH of the medium is adjusted to approximately 7.5, so it appears orange/red with a phenol red pH indicator. These types of carbohydrate fermentation tubes are therefore called phenol red (sugar) broths. For example, if the fermentation test is being done to test fermentation of glucose, glucose is added to phenol red medium and the medium is called phenol red glucose. If the fermentation test is being done to test fermentation of lactose, lactose is added to phenol red medium and the medium is called phenol red lactose.
If the carbohydrate in the medium is fermented and acidic end products are formed, the color of the medium changes from red-orange to yellow. Occasionally, bacteria will not ferment the carbohydrate, but instead will break down proteins producing ammonia (NH3) in the growth medium. In this case, the medium will become more alkaline and appear red.
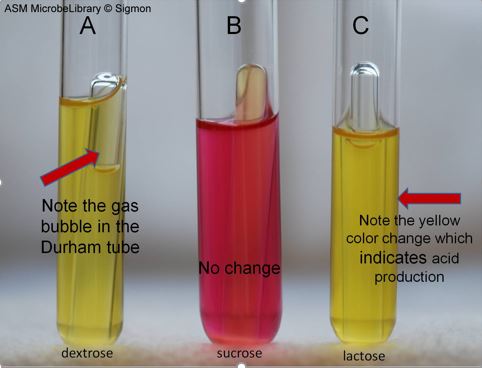
Figure 4: Fermentation reactions produced by Escherichia coli in phenol red sugar broths containing dextrose, sucrose, and lactose. Image by Janie Sigmon, York Technical College, Rock Hill, SC.
Results of a fermentation can be interpreted as follows:
- red-orange color indicates no acid was produced
- yellow color indicates acid was produced during fermentation
- a gas bubble trapped in the Durham tube indicates gas was produced during fermentation
- no gas bubble trapped in the Durham tube indicates no gas was produced
- if medium is red-orange and no gas bubble is trapped in the Durham tube, no fermentation occurred

Interpret the result of this fermentation test:
- Did this bacterial species produce gas?
- Did this bacterial species produce acid?
- Did fermentation occur?

Interpret the result of this fermentation test:
- Did this bacterial species produce gas?
- Did this bacterial species produce acid?
- Did fermentation occur?
Laboratory Instructions
Fermentation Test
In this experiment, fermentation of two different carbohydrates will be tested: glucose and lactose.
- Label three phenol red glucose tubes each with a species names to be tested (Escherichia coli, Bacillus subtilis, and Proteus vulgaris), group name, and medium name. Repeat for three phenol red lactose tubes.
- Use aseptic technique to transfer a loop of the appropriate cultures to each of the culture broths.
- Incubate cultures at 37 °C for 48 hours.
Results & Questions
|
medium color with glucose (red or yellow) |
bubble in Durham tube with glucose (+/-) |
acid production with glucose (+/-) |
gas production with glucose (+/-) |
fermentation of glucose (+/-) |
|
|---|---|---|---|---|---|
| Escherichia coli | |||||
| Bacillus subtilis | |||||
| Proteus vulgaris |
|
medium color with lactose (red or yellow) |
bubble in Durham tube with lactose (+/-) |
acid production with lactose (+/-) |
gas production with lactose (+/-) |
fermentation of lactose (+/-) |
|
|---|---|---|---|---|---|
| Escherichia coli | |||||
| Bacillus subtilis | |||||
| Proteus vulgaris |
- Complete the tables above based on the results observed from the fermentation tests.
- Was there a difference in fermentation/fermentation products produced by E. coli with glucose versus lactose? Explain your answer.
- Was there a difference in fermentation/fermentation products produced by B. subtilis with glucose versus lactose? Explain your answer.
- Was there a difference in fermentation/fermentation products produced by P. vulgaris with glucose versus lactose? Explain your answer.
- What is fermentation?
- Do all bacterial species ferment in the same way and produce the same end products? Explain your answer.
- Why is fermentation an important process in some bacterial species?
- Are fermentation tests useful for bacterial species identification and characterization? Explain your answer.
- What is the purpose of phenol red in the fermentation medium?
- What is the purpose of the Durham tube in the fermentation tube?
Attributions
- Chapter Image: MB352 General Microbiology Laboratory 2021 (Lee) by Alice_Lee@ncsu.edu is licensed under CC BY-NC-SA 4.0
- Laboratory Exercises in Microbiology: Discovering the Unseen World Through Hands-On Investigation by Susan McLaughlin and Joan Petersen is licensed under CC BY-NC
- MB352 General Microbiology Laboratory 2021 (Lee) by Alice_Lee@ncsu.edu is licensed under CC BY-NC-SA 4.0
- Red Mountain Microbiology by Jill Raymond Ph.D.; Graham Boorse, Ph.D.; Anne Mason M.S. is licensed under CC BY-NC 4.0


