1.21: Bacterial Oxygen Requirements
- Page ID
- 79453
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Explain how aerobic and anaerobic respiration differ.
- Define and recognize descriptions of obligate aerobes, obligate anaerobes, facultative anaerobes, microaerophiles, and aerotolerant anaerobes.
- Tell that bacterial species' oxygen requirements are useful for species identification and characterization.
- Describe how thioglycollate agar tubes work and how they can be used to determine bacterial species' oxygen requirements.
- Tell how anaerobic chambers and GasPak systems work to culture obligate anaerobe species.
- Successfully conduct and interpret thyioglycollate cultures to determine oxygen requirements of bacteria.
- Successfully grow bacteria in aerobic and anaerobic conditions and interpet the results.
O2 and Bacterial Metabolism & Growth
Bacteria can differ dramatically in their ability to utilize oxygen (O2). Under aerobic conditions, if the bacterial species can conduct aerobic respiration, oxygen acts as the final electron acceptor for the electron transport chain located in the plasma membrane of prokaryotes. Bacteria use the electron transport chain to generate a H+ gradient that is used by ATP synthase to make ATP. ATP is the energy source for most cellular processes and therefore essentially for keeping cells alive. In the absence of oxygen (O2), some bacteria can use alternative metabolic pathways including anaerobic respiration and/or fermentation. During anaerobic respiration, other alternative molecules are used as the final electron acceptor for the electron transport chain such as nitrate (NO3), sulfate (SO4), and carbonate (CO3).
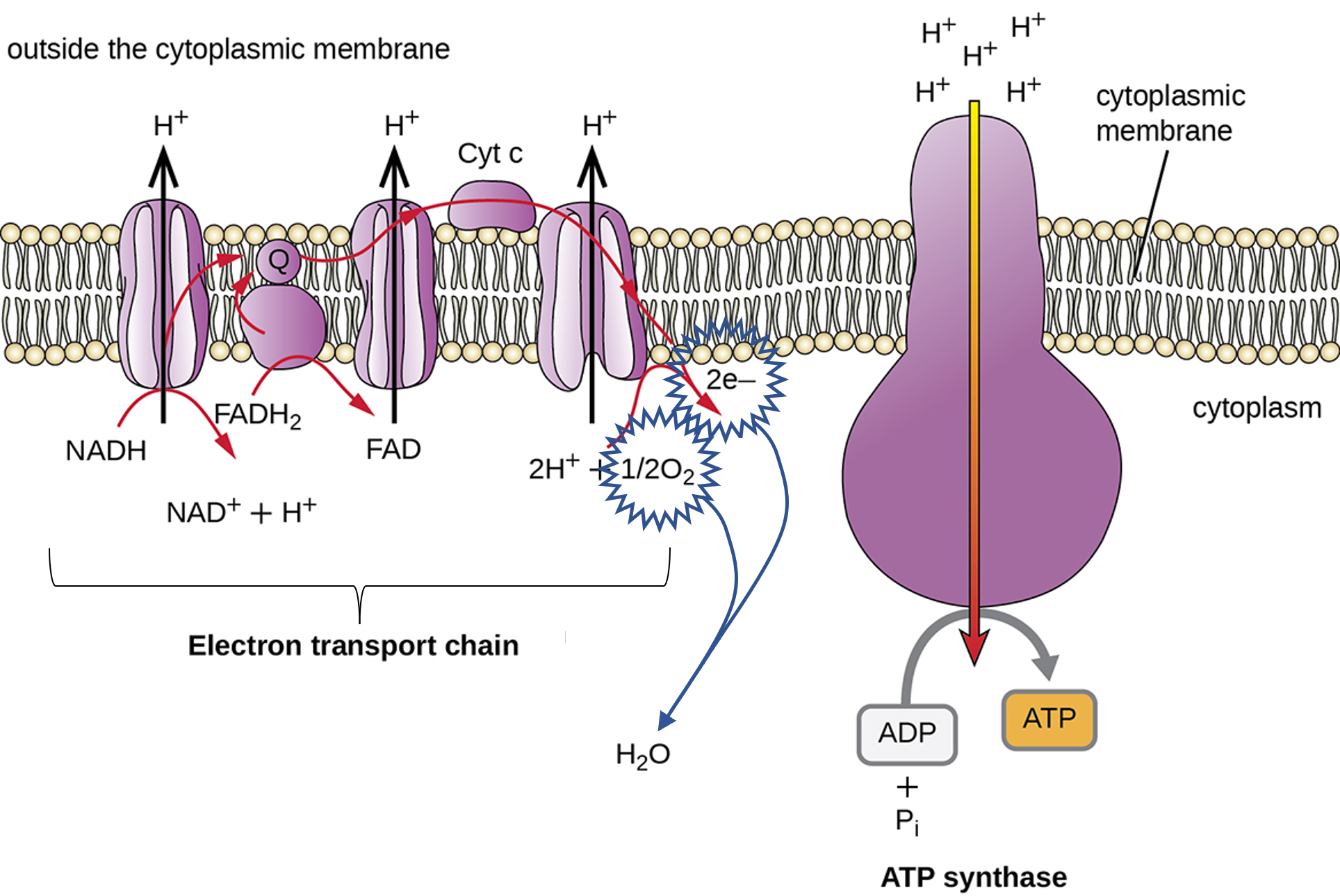
Figure 1: The electron transport chain and oxidative phosphorylation in aerobic respiration. This process is aerobic because oxygen (O2) is the final electron acceptor and combines with electrons from the electron transport chain to remove them. The electron transport chain and oxidative phosphorylation still occur for bacterial species capable of anaerobic respiration, but instead of using oxygen as the final electron acceptor, they use a different molecule (the molecule used as the final electron acceptor depends on the species but examples include nitrate, NO3, sulfate, SO4, and carbonate, CO3).
The presence or absence of molecular oxygen can be a critical factor in the ability of bacteria to grow in each environment. When bacteria use oxygen in cellular respiration and other chemical reactions, toxic superoxide and peroxides are produced. These highly reactive byproducts damage the cell unless they are quickly neutralized. Aerobic bacteria (grow in O2 environments) produce enzymes such as catalase, peroxidase and/or superoxide dismutase that break down toxic forms of oxygen and their intermediate byproducts. Bacteria called anaerobes produce ATP via anaerobic means (anaerobic respiration and/or fermentation). Anaerobes have no tolerance for oxygen since they cannot produce catalase, peroxidase and/or superoxide dismutase to remove toxic byproducts of O2.
Bacterial species are classified by their oxygen requirements as follows:
- obligate aerobes: Produce ATP via aerobic respiration. Require around 20% atmospheric oxygen.
- microaerophiles: Produce ATP via aerobic respiration or fermentation. Require between 5-15% atmospheric oxygen for growth.
- aerotolerant anaerobes: Produce ATP via anaerobic respiration and can conduct fermentation. Oxygen can be present, but they do not utilize it for ATP production or fermentation.
- facultative anaerobes: Produce ATP via aerobic respiration, anaerobic respiration, and/or fermentation. These organisms grow equally well in aerobic or anaerobic environments.
- obligate anaerobes: Produce ATP via anaerobic respiration or fermentation. These bacteria die in the presence of O2 because they lack the enzymes needed to break down toxic forms of oxygen and their intermediate byproducts.
Since bacterial species differ in their oxygen requirements, testing this feature is useful for identifying and characterizing bacterial species.
Determining Bacterial Oxygen Requirements with Thioglycollate Medium
Thioglycollate is a medium designed to test the aerotolerance (tolerance to O2) of bacteria. Along with nutrients, it contains a reducing agent, sodium thioglycollate, which combines with oxygen to produce water. Thioglycollate also contains a small amount of agar which helps reduce oxygen diffusion and helps maintain the stratification of organisms growing in different layers of the broth. Because the thioglycollate can eliminate the oxygen in the bottom of the tube, but not at the surface, varying concentrations of oxygen are found within the tube. On occasion, an indicator is added to the media to indicate the presence or absence of oxygen and shows where the aerobic and anaerobic zones separate. For example, resazurin is pink in the presence of oxygen and colorless when reduced.
One can determine a bacterium's oxygen requirements by cultivating them in a special medium called thioglycollate agar tubes. Based on the location and distribution of the bacteria in these tubes, a species can be classified as obligate aerobe, microaerophile, facultative anaerobe, aerotolerant anaerobe, or obligate anaerobe.
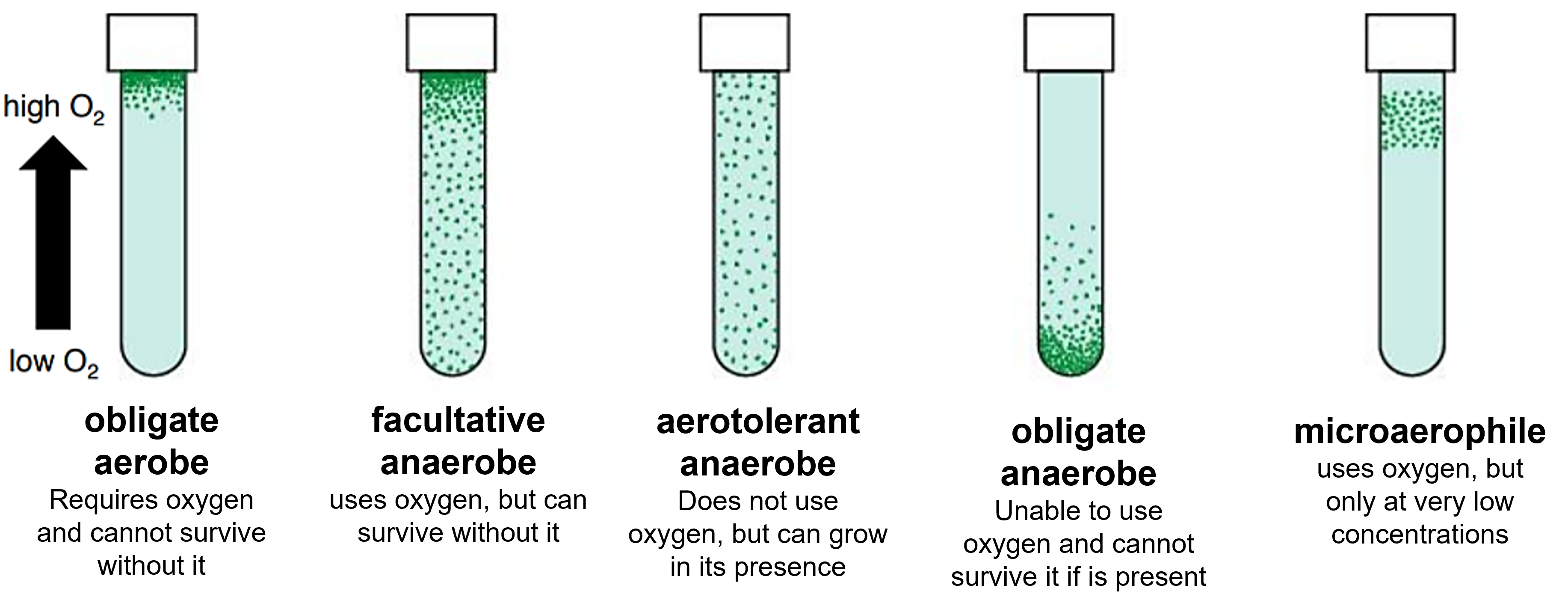
Figure 2: Microbial oxygen requirements can be determined using thioglycollate agar tubes. The green dots in this diagram represent bacterial colonies within in the agar or on its surface. The surface of the agar tube is directly exposed to atmospheric oxygen, and will be aerobic. The oxygen content of the thioglycollate medium decreases with depth until the medium becomes anaerobic towards the bottom of the tube.
Growing Bacteria in Strict Anaerobic Conditions
Anaerobic Chambers
The cultivation of anaerobic bacterial species requires an anaerobic chamber. This is a special chamber is a closed environment without O2 where the microbiologist can work with and cultivate obligate anaerobes without exposing them to oxygen. Anaerobic chambers contain a hydrogen (H2) gas mixture that is circulated through a heated palladium catalyst to remove oxygen (O2) by forming water (H2O). Anaerobic chambers use a gas mixture of H2 and nitrogen gas (N2) (5/95%) or N2/carbon dioxide (CO2)/H2 (85/10/5 %) to remove oxygen. An airlock is used to reduce O2 levels prior to the transfer of samples in and out of the chamber.
.jpg?revision=1&size=bestfit&width=660&height=556)
Figure 3: A microbiologist using an anaerobic chamber. Gloves reach into the chamber from outside of the chamber to conduct culture transfers and work with anaerobic bacteria. Inside of the anaerobic chamber there is a non-O2 atmosphere and a catalyst system to remove any O2 that might enter the chamber when samples and tools are transferred in and out of the chamber.
GasPak Anaerobic Systems
A Gas Pak jar or bag is an alternative way to grow strict anaerobes that must be grown in an atmosphere without oxygen. Plates or tubes are placed in a sealed jar or bag along with a GasPak envelopes that functions as a hydrogen and carbon dioxide generator. The hydrogen combines with the oxygen in the jar to produce water. A palladium catalyst in the chamber or bag catalyzes the formation of water from hydrogen and oxygen, thereby removing the O2. To insure anaerobic conditions are effectively produced in the GasPak jar or bag, a strip of paper soaked in methylene blue dye is included in the jar or bag. Methylene blue is colorless in an anaerobic environment and blue in an aerobic environment. These systems are compact, easy to use, and less expensive than an anaerobic chamber.

Figure 4: An anaerobic jar for cultivating obligate anaerobic species. Plates and test tubes with cultures to be cultivated in an anaerobic environment are added to the jar with a gas-generating pouch. The jar is then sealed. The gas generator pouch removes O2 inside the jar. The jar environment then remains free of O2 until the jar is opened.
Laboratory Instructions
Determining Bacterial Oxygen Requirements with Thioglycollate Medium
- The thioglycollate broth should be either boiled first before inoculation OR recently made so that the oxygen content is very low. (Your instructor will tell you if it needs to be boiled).
- Use tape to label the test tube with your group name, the bacterial species, and the type of medium.
- Inoculate a tube of thioglycollate broth with the assigned bacterial species. Make sure that the loop or needle goes down to the very bottom of the broth, but do not get the metal holder region of the loop in the sterile broth since it will contaminate it.
- Incubate at 25 °C or 37 °C.
Growing Bacteria With & Without O2
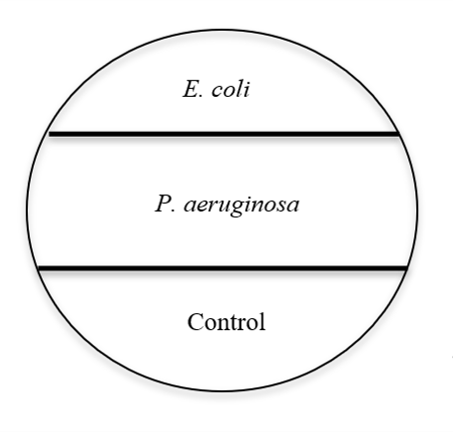
- On the underside of two TSA Petri plates, use a marker to create 3 sections on the Petri plate (see image above) with the sections labeled as Escherichia coli, Pseudomonas aeruginosa, and Control. Depending on the circumstances, your instructor may also choose to include another section with a Clostridium species as well. If that is the case, create an additional section and label it with the Clostridium species name. Label one plate as "aerobic" and the other plate as "anaerobic."
- Aseptically inoculate each section of the petri plates with the corresponding bacterial species. You may use a streak in a straight line or a zig-zag pattern within the labeled section for each. Leave the Control section without any added bacteria.
- Incubate the aerobic plate as usual at 37 °C. The anaerobic plate will be placed in an anaerobic jar or anaerobic bag and a GasPak pouch. THe GasPak will be activated by your instructor to generate an anaerobic environment and then incubated at 37 °C.
Results & Discussion
Determining Bacterial Oxygen Requirements with Thioglycollate Medium
|
|
growth in the aerobic region of the thioglycollate medium (+/-) |
growth in the microaerobic region of the thioglycollate medium (+/-) |
growth in the anaerobic region of the thioglycollate medium (+/-) |
distribution of growth in the medium (even, uneven); if uneven, where was most of the growth? | classify this species by its oxygen requirement (e.g. obligate aerobe, facultative anaerobe, etc.) |
|---|---|---|---|---|---|
|
Escherichia coli |
|
|
|||
|
Pseudomonas aeruginosa |
|
|
|||
|
Clostridium sp. (if used) |
- Examine the thioglycollate medium. Do not shake or stir. Complete the table above to summarize results and determine the oxygen requirement for each species.
- Explain how these results relate to aerobic respiration and anaerobic respiration?
- How does the thioglycollate test tube create aerobic, microaerobic, and anaerobic conditions to test oxygen tolerances of bacterial species?
- True or False. All bacterial species can grow in the presence of oxygen. Explain your answer.
- True or False. All bacterial species can grow in the absence of oxygen. Explain your answer.
- Can testing the oxygen tolerances of bacterial species be useful for species identification and characterization? Explain your answer.
Growing Bacteria With & Without O2
|
|
aerobic growth (+/-) |
anaerobic growth (+/-) |
classify this species by its oxygen requirement (e.g. obligate aerobe, facultative anaerobe, etc.) |
|---|---|---|---|
|
Escherichia coli |
|
|
|
|
Pseudomonas aeruginosa |
|
|
|
|
Clostridium sp. (if used) |
- Examine the aerobic and anaerobic petri plates and fill out the table above with the results.
- Explain how these results relate to aerobic respiration and anaerobic respiration?
- True or False. All bacterial species can grow in the presence of oxygen. Explain your answer.
- True or False. All bacterial species can grow in the absence of oxygen. Explain your answer.
- How does the GasPak system produce an anaerobic environment?
- Can testing the oxygen tolerances of bacterial species be useful for species identification and characterization? Explain your answer.
Attributions
- Anaerobic chamber (6790409341).jpg by Oak Ridge National Laboratory is licensed under CC BY 2.0
- Chapter Image: Microbiology Labs I by Delmar Larsen and Jackie Reynolds
- MB352 General Microbiology Laboratory 2021 (Lee) by Alice_Lee@ncsu.edu is licensed under CC BY-NC-SA 4.0
- Microbiology by OpenStax is licensed under CC BY 4.0
- Red Mountain Microbiology by Jill Raymond Ph.D.; Graham Boorse, Ph.D.; Anne Mason M.S. is licensed under CC BY-NC 4.0


