12.3F: B-Lymphocytes (B-Cells)
- Page ID
- 3299
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Describe the overall function of B-lymphocytes and their activation by T-dependent antigens in terms of the following:
- the antigen receptor on their surface
- how they "process" exogenous antigens
- the type of MHC molecule to which they attach peptides
- the role of lysosomes in binding of peptides from exogenous antigens by MHC-II molecules.
- the type of cell to which they present peptides
- the types of cells into which activated B-lymphocytes differentiate
B-lymphocytes (B-cells) are responsible for the production of antibody molecules during adaptive immunity. Antibodies are critical in removing extracellular microorganisms and toxins. B-lymphocytes refer to lymphocytes that are produced in the bone marrow and require bone marrow stromal cells and their cytokines for maturation. During its development, each B-lymphocyte becomes genetically programmed through a series of gene-splicing reactions to produce an antibody molecule with a unique specificity - a specific 3-dimensional shape capable of binding a specific epitope of an antigen (Figure \(\PageIndex{1}\)).
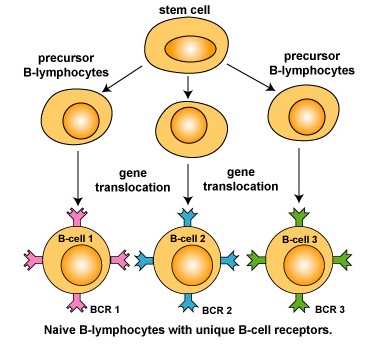
It is estimated that the human body has the ability to recognize 107 or more different epitopes and make up to 109 different antibodies, each with a unique specificity. In order to recognize this immense number of different epitopes, the body produces 107 or more distinct clones of B-lymphocytes, each with a unique B-cell receptor or BCR. In this variety of B-cell receptors there is bound to be at least one that has an epitope-binding site able to fit, at least to some degree, any antigen the immune system eventually encounters.
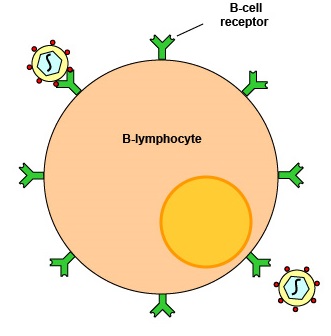
Typically, over 100,000 identical molecules of that unique antibody are placed on the surface of the B-lymphocyte where they can function as B-cell receptors capable of binding specific epitopes of a corresponding shape (Figure \(\PageIndex{2}\)). Naive B-lymphocytes can be activated by both T-dependent antigens and T-independent antigens.
Activation of naive B-lymphocytes by T-dependent antigens
In order for naive B-lymphocytes to proliferate, differentiate, and mount an antibody response against T-dependent antigens, such as most proteins, these B-lymphocytes must interact with effector T4-lymphocytes called TFH cells. All classes of antibody molecules can be made against T-dependent antigens and there is usually a memory response against such antigens.
B-Lymphocytes and T4-lymphocytes encounter antigens in secondary lymphoid organs such as the lymph nodes and the spleen. Using a lymph node as an example (Figure \(\PageIndex{3}\)A), soluble antigens, such as microbial polysaccharides and proteins and toxins, as well as microbes such as bacteria and viruses, enter the lymph node through afferent lymphatic vessels. By this time, complement pathway activation has coated these soluble antigens or microbes with opsonins such as C3b, which in turn can be degraded to C3d.
Located within the lymphoid tissues are specialized macrophages and specialized dendritic cells called follicular dendritic cells (FDCs). These macrophages have poor endocytic ability and produce few lysosomes. The FDCs are nonphagocytic. Both cell types, however, have complement receptors called CR1 and CR2 that bind to the C3b and C3d, enabling the antigens and microbes to stick to the surface of the macrophages and FDCs. However,because of the poor endocytic ability of the macrophages and the lack of endocytosis by the FDCs, the antigens and microbes are not engulfed but rather remain on the surface of the cells. In addition, the macrophages can transfer their bound antigens or microbes to FDCs (Figure \(\PageIndex{3}\)B).
Here the antigens and microbes in the lymph node can bind to complementary-shaped BCRs on naive B-lymphocytes directly, by way of macrophages, or via the FDCs (Figure \(\PageIndex{3}\)B).
Circulating naive B-lymphocytes, as a result of chemotaxis, enter lymph nodes through high endothelial venules. Any naive B-lymphocyte that bind antigens become activated and remain in the lymphoid nodes to proliferate and differentiate. Any B-lymphocytes not activated leave the lymphoid node through efferent lymphatic vessels and are returned to the bloodstream.
The first signal for the activation of a naive B-lymphocyte occurs when BCRs on the surface of the B-lymphocyte bind epitopes of antigens having a corresponding shape. A second signal is also needed for the activation of the naive B-lymphocyte. This is provided when the complement protein C3d on the microbial surface or soluble antigen binds to a complement receptor called CR2 on the surface of the naive B-lymphocyte.
Once bound, the antigen is engulfed, placed in a phagosome , and degraded with lysosomes. During this process, protein antigens are broken down into a series of peptide epitopes.These peptides eventually bind to grooves in MHC-II molecules that are then transported to the surface of the B-lymphocyte (Figure \(\PageIndex{4}\)).
Meanwhile, naïve T4-lymphocytes are being activated by epitopes of antigens bound to MHC-II molecules on antigen-presenting dendritic cells in the T-cell area of the lymph node and subsequently proliferate and differentiate into T4-effector lymphocytes such as TFH cells which remain in the lymph node. The T-cell receptors and CD4 molecules on TFH cells bind to the MHC-II molecules with bound peptide epitope on the B-lymphocyte. The binding of co-receptor molecules such as CD40L and CD28 on the surface of the effector T4-lymphocyte to the corresponding molecules CD40 and B7 on the surface of the B-lymphocyte further contribute to the interaction between these two cells (Figure \(\PageIndex{5}\)). This enables the TFH cells to produce cytokines such as interleukin-2 (IL-2) , interleukin-4 (IL-4), interleukin-5 (IL-5), and interleukin-6 (IL-6) (Figure \(\PageIndex{5}\)).
Collectively these cytokines:
- Enable activated B-lymphocytes to proliferate.
- Stimulate activated B-lymphocytes to synthesize and secrete antibodies.
- Promote the differentiation of B-lymphocytes into antibody-secreting plasma cells. See Figure \(\PageIndex{6}\).
- Enable antibody producing cells to switch the class or isotype of antibodies being produced.
|
YouTube animation illustrating production of antibodies by B-lymphocytes.
|
|
YouTube animation illustrating production of antibodies by B-lymphocytes against Streptococcus pyogenes.
|
Effector T4-lymphocytes also enable B-lymphocytes to undergo affinity maturation through a high rate of somatic mutation. This allows the B-lymphocytes to eventually "fine-tune" the shape of the antibody for better fit with the original epitope. After mutation, some antibodies fit better, some worse. To select for B-lymphocytes displaying antibodies with a better fit, the variant B-lymphocytes interact with cells called follicular dendritic cells (FDCs) in the germinal centers of the secondary lymphoid organs. The FDCs display the same antigens that activated the original B-lymphocyte. If the B-lymphocytes have high affinity antibodies for the antigen on the FDC, they are selected to survive. Those B-lymphocytes with low affinity antibodies undergo apoptosis.
With the exception of TFH cells which remain in the germinal centers of the lymph nodes and spleen, progeny of the activated B-lymphocytes and T4 effector lymphocytes leave the secondary lymphoid organs and migrate to tissues where they continue to respond to the invading antigen as long as it is present.
In the case of systemic infections or vaccinations where the antigens enter the bloodstream, plasma cells migrate to the bone marrow where antibodies can be produced for decades. After the antibodies are secreted by the plasma cells, they are found dissolved in the blood plasma and lymph. From here they can be delivered anywhere in the body via the circulatory system and the inflammatory response. In the case of infections of the mucous membranes, however, plasma cells only enter the mucous membranes where antibodies are only produced for a few months to a year or so.
During the proliferation and differentiation that follows lymphocyte activation, some of the B-lymphocytes stop replicating and become circulating, long-lived memory cells. Memory cells are capable of what is called anamnestic response or "memory", that is, they "remember" the original antigen. If that same antigen again enters the body while the B-memory cells (and T4-memory cells) are still present, these memory cells will initiate a rapid, heightened secondary response against that antigen (Figure \(\PageIndex{7}\)). This is why the body sometimes develops a permanent immunity after an infectious disease and is also the principle behind immunization.
Activation of B-lymphocytes by T-independent antigens
T-independent (TI) antigens are usually large carbohydrate and lipid molecules with multiple, repeating subunits. B-lymphocytes mount an antibody response to T-independent antigens without the requirement of interaction with effector T4-lymphocytes. Bacterial LPS from the Gram-negative cell wall and capsular polysaccharides are examples of TI antigens. The resulting antibody molecules are generally of the IgM isotype and do not give rise to a memory response. There are two basic types of T-independent antigens: TI-1 and TI-2.
a. TI-1 antigens arepathogen-associated molecular patterns or PAMPS such as lipopolysaccharide (LPS) from the outer membrane of the gram-negative cell wall and bacterial nucleic acid. These antigens activate B-lymphocytes by binding to their specific pattern-recognition receptors , in this case toll-like receptors, rather than to B-cell receptors (Figure \(\PageIndex{8}\)). Antibody molecules generated against TI-1 antigens are often called "natural antibodies" because they are always being made against bacteria present in the body.
b. TI-2 antigens, such as capsular polysaccharides, are molecules with multiple, repeating subunits. These repeating subunits activate B-lymphocytes by simultaneously cross-linking a number of B-cell receptors (Figure \(\PageIndex{9}\)).
For a Summary of Key Surface Molecules and Cellular Interactions of Naive B-Lymphocytes, see Figure \(\PageIndex{10}\).
Summary
- B-lymphocytes are responsible for the production of antibody molecules during adaptive immunity.
- Antibodies are critical in removing extracellular microorganisms and toxins.
- B-lymphocytes refer to lymphocytes that are produced in the bone marrow and require bone marrow stromal cells and their cytokines for maturation.
- During its development, each B-lymphocyte becomes genetically programmed to produce an antibody molecule with a unique 3-dimensional shape capable of binding a specific epitope of an antigen, and puts molecules of that antibody on its surface that function as B-cell receptors or BCRs.
- Naive B-lymphocytes can be activated by both T-dependent antigens and T-independent antigens.
- In order for naive B-lymphocytes to proliferate, differentiate, and mount an antibody response against T-dependent antigens, such as most proteins, these B-lymphocytes must interact with effector T4-lymphocytes called TFH cells.
- The first signal for the activation of a naive B-lymphocyte occurs when BCRs on the surface of the B-lymphocyte bind epitopes of antigens having a corresponding shape.
- Once bound to the BCR, the antigen is engulfed, placed in a phagosome, and degraded with lysosomes. During this process, protein antigens are broken down into a series of peptide epitopes, bind to MHC-II molecules, and are transported to the surface of the B-lymphocyte.
- The T-cell receptors and CD4 molecules on TFH cells bind to the MHC-II molecules with bound peptide epitope on the B-lymphocyte which enables the TFH cells to produce cytokines that collectively enable the B-lymphocytes to proliferate, synthesize and secrete antibodies, differentiate into antibody-secreting plasma cells, and switch the class of antibodies being produced.
- By way of a mutation process called affinity maturation, activated B-lymphocytes are able over time to “fine-tune" the shape of the antibody for better fit with the original epitope.
- During the proliferation and differentiation that follows lymphocyte activation, some of the B-lymphocytes stop replicating and become circulating, long-lived memory cells that will initiate a rapid, heightened secondary response against that antigen if it again enters the body.
- T-independent (TI) antigens are usually large carbohydrate and lipid molecules with multiple, repeating subunits. B-lymphocytes mount an antibody response to T-independent antigens without the requirement of interaction with effector T4-lymphocytes, but the resulting antibody molecules are generally of the IgM isotype only and do not give rise to a memory response.


