6.4A: Enrichment and Isolation
- Page ID
- 9168
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- List the growth phases of microrganisms and the different types of growth media available to culture them
The most common growth media for microorganisms are nutrient broths and agar plates; specialized media are required for some microorganisms. Some, termed fastidious organisms, require specialized environments due to complex nutritional requirements. Viruses, for example, are obligate intracellular parasites and require a growth medium containing living cells.
Growth media: defined vs. undefined
An important distinction between growth media types is that of defined versus undefined media.
A defined medium will have known quantities of all ingredients. For microorganisms, this consists of providing trace elements and vitamins required by the microbe, and especially, a defined source of both carbon and nitrogen. Glucose or glycerol is often used as carbon sources, and ammonium salts or nitrates as inorganic nitrogen sources.
An undefined medium has some complex ingredients, such as yeast extract or casein hydrolysate, which consist of a mixture of many, many chemical species in unknown proportions. Undefined media are sometimes chosen based on price and sometimes by necessity – some microorganisms have never been cultured on defined media.
Types of media
Enriched media contain the nutrients required to support the growth of a wide variety of organisms, including some of the more fastidious ones. They are commonly used to harvest as many different types of microbes as are present in the specimen. Blood agar is an enriched medium in which nutritionally-rich whole blood supplements the basic nutrients. Chocolate agar is enriched with heat-treated blood (40-45°C), which turns brown and gives the medium the color for which it is named.
Selective media are used for the growth of only selected microorganisms. For example, if a microorganism is resistant to a certain antibiotic, such as ampicillinor tetracycline, then that antibiotic can be added to the medium in order to prevent other cells, which do not possess the resistance, from growing. Media lacking an amino acid, such as proline in conjunction with E. coli unable to synthesize it, were commonly used by geneticists before the emergence of genomics to map bacterial chromosomes.

Differential/indicator media distinguish one microorganism type from another growing on the same media. This type of media uses the biochemical characteristics of a microorganism growing in the presence of specific nutrients or indicators (such as neutral red, phenol red, eosin y, or methylene blue) added to the medium to visibly indicate the defining characteristics of a microorganism. This type of media is used for the detection of microorganisms and by molecular biologists to detect recombinant strains of bacteria. The agar triple-sugar iron (TSI) is one of the culture media used for the differentiation of most enterobacteria.
Growth in closed culture systems, such as a batch culture in LB broth, where no additional nutrients are added and waste products are not removed, the bacterial growth will follow a predicted growth curve and can be modeled.
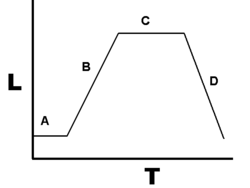
Growth phases
During lag phase, bacteria adapt themselves to growth conditions. It is the period where the individual bacteria are maturing and not yet able to divide. During the lag phase of the bacterial growth cycle, synthesis of RNA, enzymes and other molecules occurs.
Exponential phase (sometimes called the log or logarithmic phase) is a period characterized by cell doubling. The number of new bacteria appearing per unit time is proportional to the present population.Under controlled conditions, cyanobacteria can double their population four times a day. Exponential growth cannot continue indefinitely, however, because the medium is soon depleted of nutrients and enriched with wastes.
The stationary phase is due to a growth-limiting factor; this is mostly depletion of a nutrient, and/or the formation of inhibitory products such as organic acids.
At death phase, bacteria run out of nutrients and die.
Culture
Batch culture is the most common laboratory-growth method in which bacterial growth is studied, but it is only one of many. The bacterial culture is incubated in a closed vessel with a single batch of medium.
In some experimental regimes, some of the bacterial culture is periodically removed and added to fresh sterile medium. In the extreme case, this leads to the continual renewal of the nutrients. This is a chemostat, also known as an open or continuous culture: a steady state defined by the rates of nutrient supply and bacterial growth. In comparison to batch culture, bacteria are maintained in exponential growth phase, and the growth rate of the bacteria is known. Related devices include turbidostats and auxostats. Bacterial growth can be suppressed with bacteriostats, without necessarily killing the bacteria.
In a synecological culture, a true-to-nature situation in which more than one bacterial species is present, the growth of microbes is more dynamic and continual.
Key Points
- The most common growth media for microorganisms are nutrient broths and agar plates.
- Open cultures allow for a replenishment of nutrients and a reduction of waste buildup in the media.
- Selective media are used for the growth of only selected microorganisms.
- Differential media or indicator media distinguish one microorganism type from another growing on the same media.
Key Terms
- closed culture: A closed culture has no additional nutrients added to the system, and waste products are not removed. Cultures in a closed system will follow a predicted growth curve.
- Enriched media: Contains nutrients required to support the growth of a wide variety of organisms.
- open culture: A continuous culture where periodically some of the bacterial culture is removed and added to fresh sterile medium.


