4.1: Enzymes
- Page ID
- 4626
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
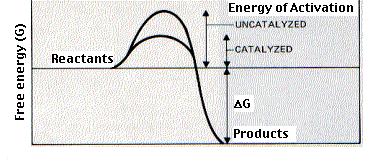
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Enzymes are catalysts. Most are proteins. (A few ribonucleoprotein enzymes have been discovered and, for some of these, the catalytic activity is in the RNA part rather than the protein part. Link to discussion of these ribozymes). Enzymes bind temporarily to one or more of the reactants — the substrate(s) — of the reaction they catalyze. In doing so, they lower the amount of activation energy needed and thus speed up the reaction.
Examples:
- Catalase catalyzes the decomposition of hydrogen peroxide into water and oxygen. \[2H_2O_2 \rightarrow 2H_2O + O_2\] One molecule of catalase can break 40 million molecules of hydrogen peroxide each second.
- Carbonic anhydrase is found in red blood cells where it catalyzes the reaction. \[CO_2 + H_2O \rightleftharpoons H^+ + HCO_3^−\] It enables red blood cells to transport carbon dioxide from the tissues to the lungs. One molecule of carbonic anhydrase can process one million molecules of CO2 each second.
- Acetylcholinesterase catalyzes the breakdown of the neurotransmitter acetylcholine at several types of synapses as well as at the neuromuscular junction — the specialized synapse that triggers the contraction of skeletal muscle. One molecule of acetylcholinesterase breaks down 25,000 molecules of acetylcholine each second. This speed makes possible the rapid "resetting" of the synapse for transmission of another nerve impulse.
Enzyme activity can be analyzed quantitatively. To do its work, an enzyme must unite — even if ever so briefly — with at least one of the reactants. In most cases, the forces that hold the substrate in the active site of the enzyme are noncovalent, an assortment of hydrogen bonds, ionic interactions, and hydrophobic interactions.
Most of these interactions are weak and especially so if the atoms involved are farther than about one angstrom from each other. So successful binding of the substrate in the active site of the enzyme requires that the two molecules be able to approach each other closely over a fairly broad surface. Thus the analogy that a substrate molecule binds its enzyme like a key in a lock. This requirement for complementarity in the configuration of substrate and enzyme explains the remarkable specificity of most enzymes. Generally, a given enzyme is able to catalyze only a single chemical reaction or, at most, a few reactions involving substrates sharing the same general structure.
Competitive inhibition
The necessity for a close, if brief, fit between enzyme and substrate explains the phenomenon of competitive inhibition. One of the enzymes needed for the release of energy within the cell is succinic dehydrogenase. It catalyzes the oxidation (by the removal of two hydrogen atoms) of succinic acid. If one adds malonic acid to cells, or to a test tube mixture of succinic acid and the enzyme, the action of the enzyme is strongly inhibited. This is because the structure of malonic acid allows it to bind to the same site on the enzyme. But there is no oxidation so no speedy release of products. The inhibition is called competitive because if you increase the ratio of succinic to malonic acid in the mixture, you will gradually restore the rate of catalysis. At a 50:1 ratio, the two molecules compete on roughly equal terms for the binding (=catalytic) site on the enzyme.
Enzyme cofactors
Many enzymes require the presence of an additional nonprotein - a cofactor.
- Some of these are metal ions such as Zn2+ (the cofactor for carbonic anhydrase), Cu2+, Mn2+, K+, and Na+.
- Some cofactors are small organic molecules called coenzymes. The B vitamins thiamine (B1), riboflavin (B2) and nicotinamide
are precursors of coenzymes.
Coenzymes may be covalently bound to the protein part (called the apoenzyme) of enzymes as a prosthetic group. Others bind more loosely and, in fact, may bind only transiently to the enzyme as it performs its catalytic act.
A number of lysozymes are found in nature; in human tears and egg white, for examples. The enzyme is antibacterial because it degrades the polysaccharide that is found in the cell walls of many bacteria. It does this by catalyzing the insertion of a water molecule at forming a glycosidic bond. This hydrolysis breaks the chain at that point.
The bacterial polysaccharide consists of long chains of alternating amino sugars:
- N-acetylglucosamine (NAG)
- N-acetylmuramic acid (NAM)
These hexose units resemble glucose except for the presence of the side chains containing amino groups.
Lysozyme is a globular protein with a deep cleft across part of its surface. Six hexoses of the substrate fit into this cleft. With so many oxygen atoms in sugars, as many as 14 hydrogen bonds form between the six amino sugars and certain amino acid R groups such as Arg-114, Asn-37, Asn-44, Trp-62, Trp-63, and Asp-101. Some hydrogen bonds also form with the C=O groups of several peptide bonds. In addition, hydrophobic interactions may help hold the substrate in position.
X-ray crystallography has shown that as lysozyme and its substrate unite, each is slightly deformed. The fourth hexose in the chain (ring #4) becomes twisted out of its normal position. This imposes a strain on the C-O bond on the ring-4 side of the oxygen bridge between rings 4 and 5. It is just at this point that the polysaccharide is broken. A molecule of water is inserted between these two hexoses, which breaks the chain. Here, then, is a structural view of what it means to lower activation energy. The energy needed to break this covalent bond is lower now that the atoms connected by the bond have been distorted from their normal position.
As for lysozyme itself, binding of the substrate induces a small (~0.75Å) movement of certain amino acid residues so the cleft closes slightly over its substrate. So the "lock" as well as the "key" changes shape as the two are brought together. (This is sometimes called "induced fit".)
The amino acid residues in the vicinity of rings 4 and 5 provide a plausible mechanism for completing the catalytic act. Residue 35, glutamic acid (Glu-35), is about 3Å from the -O- bridge that is to be broken. The free carboxyl group of glutamic acid is a hydrogen ion donor and available to transfer H+ to the oxygen atom. This would break the already-strained bond between the oxygen atom and the carbon atom of ring 4.
Now having lost an electron, the carbon atom acquires a positive charge. Ionized carbon is normally very unstable, but the attraction of the negatively-charged carboxyl ion of Asp-52 could stabilize it long enough for an -OH ion (from a spontaneously dissociated water molecule) to unite with the carbon. Even at pH 7, water spontaneously dissociates to produce H+ and OH- ions. The hydrogen ion (H+) left over can replace that lost by Glu-35. In either case, the chain is broken, the two fragments separate from the enzyme, and the enzyme is free to attach to a new location on the bacterial cell wall and continue its work of digesting it.
Factors Affecting Enzyme Action
The activity of enzymes is strongly affected by changes in pH and temperature. Each enzyme works best at a certain pH (left graph) and temperature (right graph), its activity decreasing at values above and below that point. This is not surprising considering the importance of tertiary structure (i.e. shape) in enzyme function and noncovalent forces, e.g., ionic interactions and hydrogen bonds, in determining that shape. Examples:
- the protease pepsin works best as a pH of 1–2 (found in the stomach)
- the protease trypsin is inactive at such a low pH but very active at a pH of 8 (found in the small intestine as the bicarbonate of the pancreatic fluid neutralizes the arriving stomach contents).
Changes in pH alter the state of ionization of charged amino acids (e.g., Asp, Lys) that may play a crucial role in substrate binding and/or the catalytic action itself. Without the unionized -COOH group of Glu-35 and the ionized -COO- of Asp-52, the catalytic action of lysozyme would cease. Hydrogen bonds are easily disrupted by increasing temperature. This, in turn, may disrupt the shape of the enzyme so that its affinity for its substrate diminishes.
Regulation of Enzyme Activity
Several mechanisms work to make enzyme activity within the cell efficient and well-coordinated.
Anchoring enzymes in membranes
Many enzymes are inserted into cell membranes, for examples,
- the plasma membrane
- the membranes of mitochondria and chloroplasts
- the endoplasmic reticulum
- the nuclear envelope
These are locked into spatial relationships that enable them to interact efficiently.
Inactive precursors
Enzymes, such as proteases, that can attack the cell itself are inhibited while within the cell that synthesizes them. For example, pepsin is synthesized within the chief cells (in gastric glands) as an inactive precursor, pepsinogen. Only when exposed to the low pH outside the cell is the inhibiting portion of the molecule removed and active pepsin produced.
Feedback Inhibition
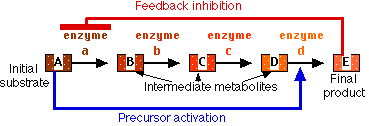
If the product of a series of enzymatic reactions, e.g., an amino acid, begins to accumulate within the cell, it may specifically inhibit the action of the first enzyme involved in its synthesis (red bar). Thus further production of the enzyme is halted.
Precursor Activation
The accumulation of a substance within a cell may specifically activate (blue arrow) an enzyme that sets in motion a sequence of reactions for which that substance is the initial substrate. This reduces the concentration of the initial substrate.
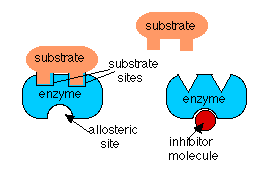
In the case if feedback inhibition and precursor activation, the activity of the enzyme is being regulated by a molecule which is not its substrate. In these cases, the regulator molecule binds to the enzyme at a different site than the one to which the substrate binds. When the regulator binds to its site, it alters the shape of the enzyme so that its activity is changed. This is called an allosteric effect. In feedback inhibition, the allosteric effect lowers the affinity of the enzyme for its substrate and in precursor activation, the regulator molecule increases the affinity of the enzyme in the series for its substrate.
Regulation of Enzyme Synthesis
The four mechanisms described above regulate the activity of enzymes already present within the cell. What about enzymes that are not needed or are needed but not present? Here, too, control mechanisms are at work that regulate the rate at which new enzymes are synthesized. Most of these controls work by turning on or off — the transcription of genes.
If, for example, ample quantities of an amino acid are already available to the cell from its extracellular fluid, synthesis of the enzymes that would enable the cell to produce that amino acid for itself is shut down. Conversely, if a new substrate is made available to the cell, it may induce the synthesis of the enzymes needed to cope with it. Yeast cells, for example, do not ordinarily metabolize lactose, and no lactase can be detected in them. However, if grown in a medium containing lactose, they soon begin synthesizing lactase — by transcribing and translating the necessary gene(s) — and so can begin to metabolize the sugar. E. coli also has a mechanism which regulates enzyme synthesis by controlling translation of a needed messenger RNA.


