15.7B: Asexual Reproduction in Animals
- Page ID
- 5497
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Asexual reproduction is the formation of new individuals from the cell(s) of a single parent. It is very common in plants; less so in animals.
Asexual Reproduction in Plants
All plant organs have been used for asexual reproduction, but stems are the most common.
Stems

In some species, stems arch over and take root at their tips, forming new plants. The horizontal above-ground stems (called stolons) of the strawberry produce new daughter plants at alternate nodes. Underground stems such as rhizomes, bulbs, corms and tubers are used for asexual reproduction as well as for food storage. Irises and day lilies, for example, spread rapidly by the growth of their rhizomes.
Leaves

The above photo shows the leaves of the common ornamental plant Bryophyllum (also called Kalanchoë). Mitosis at meristems along the leaf margins produce tiny plantlets that fall off and can take up an independent existence.
Roots
Some plants use their roots for asexual reproduction. The dandelion is a common example. Trees, such as the poplar or aspen, send up new stems from their roots. In time, an entire grove of trees may form - all part of a clone of the original tree.
Plant Propagation
Commercially-important plants are often deliberately propagated by asexual means in order to keep particularly desirable traits (e.g., flower color, flavor, resistance to disease). Cuttings may be taken from the parent and rooted.
Grafting

Grafting is widely used to propagate a desired variety of shrub or tree. All apple varieties, for example, are propagated this way.
Apple seeds are planted only for the root and stem system that grows from them. After a year's growth, most of the stem is removed and a twig (scion) taken from a mature plant of the desired variety is inserted in a notch in the cut stump (the stock). So long the cambiums of scion and stock are united and precautions are taken to prevent infection and drying out, the scion will grow. It will get all its water and minerals from the root system of the stock. However, the fruit that it will eventually produce with be identical (assuming that it is raised under similar environmental conditions) to the fruit of the tree from which the scion was taken.
Apomixis
Citrus trees and many other species of angiosperms use their seeds as a method of asexual reproduction; a process called apomixis.
- In one form, the egg is formed with 2n chromosomes and develops without ever being fertilized.
- In another version, the cells of the ovule (2n) develop into an embryo instead of - or in addition to - the fertilized egg.
Hybridization between different species often yields infertile offspring. But in plants, this does not necessarily doom the offspring. Many such hybrids use apomixis to propagate themselves. The many races of Kentucky bluegrass growing in lawns across North America and the many races of blackberries are two examples of sterile hybrids that propagate successfully by apomixis. Recently, an example of apomixis in gymnosperms was discovered (see Pichot, C., et al, in the 5 July 2001 issue of Nature). In a rare cypress, the pollen grains are diploid, not haploid, and can develop into an embryo when they land on either
- the female cones of their own species (rare) or
- those of a much more common species of cypress.
Is this paternal apomixis in a surrogate mother a desperate attempt to avoid extinction?
Breeding apomictic crop plants
Many valuable crop plants (e.g., corn) cannot be propagated by asexual methods like grafting.
Agricultural scientists would dearly love to convert these plants to apomixis: making embryos that are genetic clones of themselves rather than the product of sexual reproduction with its inevitable gene reshuffling. After 20 years of work, an apomictic corn (maize) has been produced, but it does not yet produce enough viable kernels to be useful commercially.
Asexual Reproduction in Animals
Budding
Here, offspring develop as a growth on the body of the parent. In some species, e.g., jellyfishes and many echinoderms, the buds break away and take up an independent existence. In others, e.g., corals, the buds remain attached to the parent and the process results in colonies of animals. Budding is also common among parasitic animals, e.g., tapeworms.
Fragmentation
As certain tiny worms grow to full size, they spontaneously break up into 8 or 9 pieces. Each of these fragments develops into a mature worm, and the process is repeated.
Parthenogenesis
In parthenogenesis ("virgin birth"), the females produce eggs, but these develop into young without ever being fertilized. Parthenogenesis occurs in some fishes, several kinds of insects, and a few species of frogs and lizards. It does not normally occur in mammals because of their imprinted genes. However, using special manipulations to circumvent imprinting, laboratory mice have been produced by parthenogenesis.
In a few nonmammalian species it is the only method of reproduction, but more commonly animals turn to parthenogenesis only under certain circumstances. Examples:
- Aphids use parthenogenesis in the spring when they find themselves with ample food. In this species, reproduction by parthenogenesis is more rapid than sexual reproduction, and the use of this mode of asexual reproduction permits the animals to quickly exploit the available resources.
- Female Komodo dragons (the largest lizard) can produce offspring by parthenogenesis when no male is available for sexual reproduction. Their offspring are homozygous at every locus including having identical sex chromosomes. Thus the females produce all males because, unlike mammals, females are the heterogametic sex (ZW) while males are homogametic (ZZ).
Parthenogenesis is forced on some species of wasps when they become infected with bacteria (in the genus Wolbachia). Wolbachia can pass to a new generation through eggs, but not through sperm, so it is advantageous to the bacterium for females to be made rather that males. In these wasps (as in honeybees), fertilized eggs (diploid) become females and unfertilized (haploid) eggs become males. However, in Wolbachia-infected females, all their eggs undergo endoreplication producing diploid eggs that develop into females without fertilization, by parthenogenesis. Treating the wasps with an antibiotic kills off the bacteria and "cures" the parthenogenesis!
Occasionally worker honeybees develop ovaries and lay unfertilized eggs. Usually these are haploid, as you would expect, and develop into males. However, workers of the subspecies Apis mellifera capensis (the Cape honeybee) can lay unfertilized diploid eggs that develop into females (who continue the practice). The eggs are produced by meiosis, but then the polar body nucleus fuses with the egg nucleus restoring diploidy (2n). The phenomenon is called automictic thelytoky.

Cape honey bees gorging on honey. (CC BY-SA 3.0; Discott)
Why Choose Asexual Reproduction?
Perhaps the better question is: Why not? After all, asexual reproduction would seem a more efficient way to reproduce. Sexual reproduction requires males but they themselves do not produce offspring. Two general explanations for the overwhelming prevalence of sexually-reproducing species over asexual ones are:
- Perhaps sexual reproduction has kept in style because it provides a mechanism to weed out (through the recombination process of meiosis) harmful mutations that arise in the population reducing its fitness. Asexual reproduction leads to these mutations becoming homozygous and thus fully exposed to the pressures of natural selection.
- Perhaps it is the ability to adapt quickly to a changing environment that has caused sex to remain the method of choice for most living things.
Purging Harmful Mutations
Most mutations are harmful - changing a functional allele to a less or nonfunctional one. An asexual population tends to be genetically static. Mutant alleles appear but remain forever associated with the particular alleles present in the rest of that genome. Even a beneficial mutation will be doomed to extinction if trapped along with genes that reduce the fitness of that population. But with the genetic recombination provided by sex, new alleles can be shuffled into different combinations with all the other alleles available to the genome of that species. A beneficial mutation that first appears alongside harmful alleles can, with recombination, soon find itself in more fit genomes that will enable it to spread through a sexual population.
Evidence (from Paland and Lynch in the 17 February 2006 issue of Science): Some strains of the water flea Daphnia pulex (a tiny crustacean) reproduce sexually, others asexually. The asexual strains accumulate deleterious mutations in their mitochondrial genes four times as fast as the sexual strains.
Evidence (from Goddard et al. in the 31 March 2005 issue of Nature): Budding yeast missing two genes essential for meiosis adapt less rapidly to growth under harsh conditions than an otherwise identical strain that can undergo genetic recombination. Under good conditions, both strains grow equally well.
Evidence (from Rice and Chippindale in the 19 October 2001 issue of Science):
Using experimental Drosophila populations, they found that a beneficial mutation introduced into chromosomes that can recombine did over time increase in frequency more rapidly than the same mutation introduced into chromosomes that could not recombine.
So sex provides a mechanism for testing new combinations of alleles for their possible usefulness to the phenotype:
- deleterious alleles weeded out by natural selection
- useful ones retained by natural selection
Some organisms may still gain the benefits of genetic recombination while avoiding sex. Many mycorrhizal fungi use asexual reproduction only. However, at least two species have been shown to have multiple — similar — copies of the same gene; that is, are polyploid. Perhaps recombination between these (during mitosis?) enables these organisms to avoid the hazards of accumulating deleterious mutations. (See the paper by Pawlowska and Taylor in the 19 Feb 2004 issue of Nature.) But there are many examples of populations that thrive without sex, at least while they live in a stable environment.
Red Queen hypothesisnment
As we have seen (above), populations without sex are genetically static. They may be well-adapted to a given environment, but will be handicapped in evolving in response to changes in the environment. One of the most potent environmental forces acting on a species environment is its parasites. The speed with which parasites like bacteria and viruses can change their virulence may provide the strongest need for their hosts to have the ability to make new gene combinations. So sex may be virtually universal because of the never-ending need to keep up with changes in parasites.
Evidence:
- Some parasites interfere with sexual reproduction in their host:
- Wolbachia-induced parthenogenesis discussed above is an example.
- Several types of fungi block wind pollination of their grass hosts forcing them to inbreed with its resulting genetic uniformity.
- There is some evidence that genetically uniform populations are at increased risk of devastating epidemics and population crashes.
- Flour beetles (Tribolium castaneum) parasitized by the microsporidium Nosema whitei increase the rate of recombination during meiosis.
- Drosophila females parasitized by bacteria produce more recombinant offspring than non-infected mothers do.
The idea that a constantly-changing environment, especially with respect to parasites, drives evolution is often called the Red Queen hypothesis. It comes from Lewis Carroll's book Through the Looking Glass, where the Red Queen says "Now here, you see, it takes all the running you can do to keep in the same place".
The possibilities outlined above are not mutually exclusive and a recent study [see Morran, L. T., et al., in Nature, 462:350, 19 November 2009] suggests that both forces are at work in favoring sexual reproduction over its alternatives.
The organism for testing these theories was Caenorhabditis elegans. While C. elegans does not reproduce asexually, most worms are hermaphrodites and usually reproduce by self-fertilization with each individual fertilizing its own eggs. This quickly results in its genes becoming homozygous and thus fully-exposed to natural selection just as they are in asexually-reproducing species. Hermaphrodites have two X chromosomes and self-fertilization ("selfing") usually produces more of the same; that is, hermaphrodites produce more hermaphrodites. However, an occasional nondisjunction generates an embryo with a single X chromosome and this develops into a male. These males can mate with hermaphrodites (their sperm is preferred over the hermaphrodites own) and, in fact, such "outcrossing" produces a larger number of offspring. It also produces 50% hermaphrodites and 50% males.
Testing the role of outcrossing vs. self-fertilization in maintaining fitness in the face of an increased mutation rate
These workers developed six strains of worms:
- two that could reproduce only by selfing
- two that could reproduce only by crossing a male with an hermaphrodite ("outcrossing")
- "wild-type" worms
All the strains were exposed to a chemical mutagen that increased the spontaneous mutation rate some fourfold.
The results: After 50 generations, the
- the strains of worms that could reproduce only by selfing suffered a serious decline in fitness
- the strains of worms that could reproduce only by outcrossing suffered no decline
- the wild-type worms with intermediate levels of outcrossing (20–30%) suffered only moderate declines in fitness
Fitness was measured by placing the worms in a petri dish with a barrier that they had to cross to reach their food (E. coli).
The conclusion: the genetic recombination provided by outcrossing protected the worms from loss of fitness even in the face of an increase in mutation rate.
Testing the role of outcrossing vs. self-fertilization in the speed of adaptation to a changed environment
For these tests, one of each category of mating types was exposed over 40 generations to a pathogenic bacterium (Serratia marcescens) that killed most worms when eaten by them.
The results: After 40 generations,
- the strain of worms that could reproduce only by selfing were just as susceptible to the pathogen as they were at the start while
- the strain of worms that could reproduce only by outcrossing had evolved a high degree of resistance to the pathogen
- the wild-type worms only developed a modest increase in their resistance to the bacteria.
Since these studies were reported, the same team has expanded their experiments to examine the effects of evolution in the pathogen (Serratia marcescens), that is, to look for evidence of coevolution of host and parasite. (Reported by Morran, L. T., et al., in Science, 333: 216, 8 July 2011.)
Over 30 generations of worms, they harvested and tested the bacteria recovered from the bodies of worms that had died within 24 hours of infection. They found that:
- Worms that can maintain genetic variability by outcrossing suffered substantially lower mortality from the coevolved parasite that did worms from the starting population (kept frozen until used).
- Worms that could only reproduce by selfing became so susceptible to the evolving strain of Serratia marcescens that they died out within 20 generations.
- Curiously, the selection pressure of the increasing virulence of Serratia marcescens caused wild-type worms to increase their rate of outcrossing from the normal 20–30% to over 80%. So one response to the pressure of this coevolving parasite was to promote sex in its host.
Rotifers are microscopic invertebrates. They are assigned a phylum of their own (not discussed elsewhere in these pages). The phylum includes:
- a class of ~1,500 species called monogonont rotifers (they have only a single gonad). The monogonont rotifers can choose either asexual or sexual reproduction as circumstances warrant.
- a class of ~350 species called bdelloid rotifers. The bdelloid rotifers are limited to asexual reproduction only. Even after years of study, neither males nor haploid eggs have ever been found in any members of this group. It looks as though they gave up sexual reproduction millions of years ago.
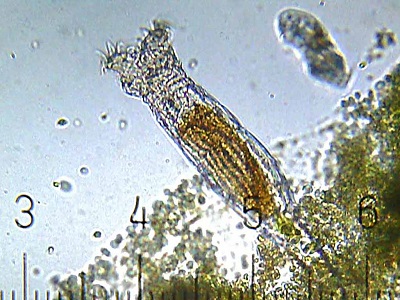
A bdelloid rotifer. (CC BY-SA 3.0; Bob Blaylock)
Laboratory studies show that monogonont rotifers favor asexual reproduction when they are living in a stable environment but shift to more sexual reproduction when placed in a varied or unfavorable environment. As they adapt to the new environment, they gradually switch back to asexual reproduction.
But how have the bdelloid rotifers that never engage in sexual reproduction managed to survive? How have they avoided the demands of the Red Queen; that is, avoided extinction at the hands of parasites?
One study (Wilson, C. G. and Sherman, P. W., Science, 327:574, 29 January 2010) reveals a mechanism. These tiny animals can be completely desiccated (dried out) and remain in suspended animation for years. In the desiccated state, they can be blown vast distances (some species are worldwide in their distribution). Once deposited in a moist environment (a few drops of water are sufficient), they resume an active life. Wilson and Sherman have shown that the desiccation that is harmless to the rotifers is lethal to their fungal parasite. So once dried, they are not only cured of their parasite, but can then be blown to a spot where they can resume an active life with no parasites present.
Another way in which these rotifers can avoid the evolutionary dead-end expected of asexually-reproducing organisms has been revealed by DNA sequencing of their genome. It turns out that they can purge their genome of deleterious alleles by gene conversion (during mitosis).
In any case despite its disadvantages sexual reproduction is here to stay
- reducing the effect of harmful mutations
- increasing the speed with which populations can adapt to changes in their environment
Contibutors
John W. Kimball. This content is distributed under a Creative Commons Attribution 3.0 Unported (CC BY 3.0) license and made possible by funding from The Saylor Foundation.
\


