24.6: Cycles of Matter
- Page ID
- 17819
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Did a dinosaur once drink the water in Figure \(\PageIndex{1}\)? You may have heard the claim — usually made in the context of the water cycle — that the same water exists on Earth now as existed tens of millions of years ago when dinosaurs roamed the planet. In fact, dinosaurs probably did not once drink the same water molecules that we consume today. Whereas the atoms that make up water molecules have existed for eons, individual water molecules are broken down and reformed too often to last that long. Nonetheless, unlike energy, which is continuously added to Earth by the sun, water is constantly recycled.

Biogeochemical Cycles
The water and chemical elements that organisms need continuously cycle through ecosystems, passing repeatedly through their biotic and abiotic components. These cycles are called biogeochemical cycles because they are cycles of chemicals that include both organisms (bio) and abiotic components such as the ocean or rocks (geo). As matter moves through a biogeochemical cycle, it may be held for various periods of time in different components of the cycle. A component of a biogeochemical cycle that holds an element or water for a long period of time is called a reservoir. For example, the deep ocean is a reservoir for water. It may hold water for thousands of years.
The rest of this concept takes a closer look at four particular biogeochemical cycles: the water, carbon, and nitrogen cycles
Water Cycle
Water is essential to all living things on Earth because virtually all biochemical reactions take place in water. Water can dissolve almost anything, so it also provides an efficient way to transfer substances between and within cells. The water cycle, also known as the hydrological cycle, describes the continuous movement of water on, above, and below Earth’s surface. As it cycles, water moves from one exchange pool or reservoir to another. In different parts of the cycle, water exists as a liquid (water), solid (ice), or gas (water vapor). Therefore, the water cycle includes several physical processes by which water changes state.
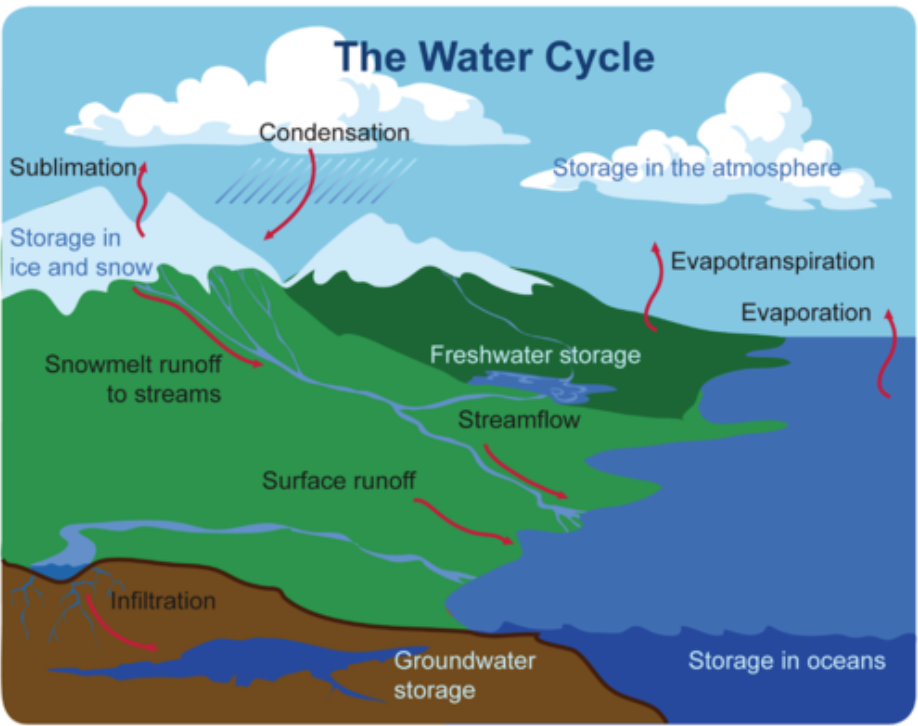
Movement through the water cycle
- Evaporation occurs when water on Earth’s surface changes to water vapor. When the sun heats water, it gives water molecules enough energy to escape into the atmosphere.
- Sublimation occurs when ice and snow change directly to water vapor without first melting to form liquid water. Sublimation occurs because of the heat from the sun.
- Transpiration occurs when plants release water vapor through leaf pores called stomata. Plants take up more water through their roots than they need for photosynthesis and other processes. Much of this excess water is given off via transpiration.
- Condensation is the process in which water vapor changes to liquid water, forming water droplets. If enough water droplets are present, they may form a visible cloud. If the droplets become large enough, they fall to Earth because of gravity as precipitation, such as rain, snow, sleet, or hail.
- Precipitation that falls on land may flow over the surface of the ground. This water is called runoff, and it may eventually flow into a body of water.
- Some of the precipitation that falls on land may soak into the ground and become groundwater. Groundwater may seep out of the ground at a spring or into a body of water such as a lake or the ocean. Some groundwater may be taken up by plant roots. Some may flow deeper underground to an aquifer.
Carbon Cycle
Carbon is the basis of life on Earth. Chains of carbon bonded together to form the backbone of many biochemical molecules. Carbon is also an important component of rocks and minerals, and carbon exists in the atmosphere in compounds such as carbon dioxide. The carbon cycle is the biogeochemical cycle in which carbon moves through the biotic and abiotic components of ecosystems. The carbon cycle is represented by the diagram in Figure \(\PageIndex{3}\).
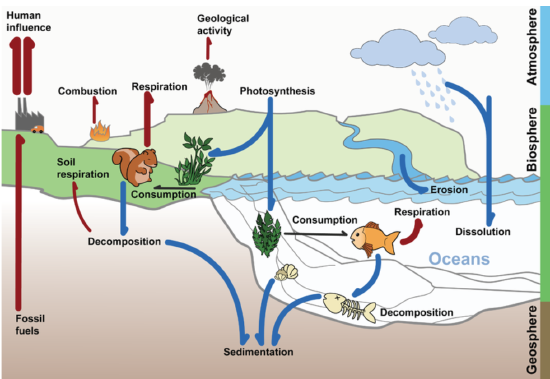
Carbon cycles quickly between organisms and the atmosphere. Cellular respiration by living things releases carbon into the atmosphere as carbon dioxide. Photosynthesis by producers such as plants removes carbon dioxide from the atmosphere and uses it to make organic carbon compounds. Carbon in organic compounds moves through ecosystem communities from producers to consumers, as modeled by food chains and food webs that show feeding relationships. Carbon is also released back into the environment when organisms decompose.
Several human actions release huge amounts of additional carbon into the atmosphere. The most significant of these actions is the burning of fossil fuels. Large amounts of carbon in the gas methane are also released into the atmosphere from the decomposition of livestock manure and the wastes in landfills. Some natural events can also quickly add carbon to the atmosphere. Wildfires produce carbon dioxide as a product of combustion, and volcanic eruptions release carbon dioxide from molten rock (magma). Large volcanic eruptions (like the one in Figure \(\PageIndex{4}\)) can release enormous amounts of carbon dioxide in a short period of time.

Carbon generally cycles more slowly through other processes. For example, running water slowly dissolves carbon in rocks, and most of this carbon ends up in the ocean. The top layer of ocean water dissolves some carbon dioxide out of the atmosphere, and carbon also enters ocean water from the decomposition of aquatic organisms. Carbon from these sources may settle to the bottom of the ocean as sediment. Over millions of years, this carbon may form fossil fuels or carbon-containing rocks. Carbon can remain in these reservoirs for millions of years.
Nitrogen Cycle
Nitrogen makes up 78 percent of Earth’s atmosphere. It is also an important element in living things. Nitrogen is needed for proteins, nucleic acids, and many other organic molecules, including chlorophyll, without which plants and other photoautotrophs could not carry out photosynthesis. The nitrogen cycle is the biogeochemical cycle that recycles nitrogen through the biotic and abiotic components of ecosystems. Figure \(\PageIndex{5}\) shows how nitrogen cycles through a terrestrial ecosystem. Nitrogen passes through aquatic ecosystems in a similar cycle.
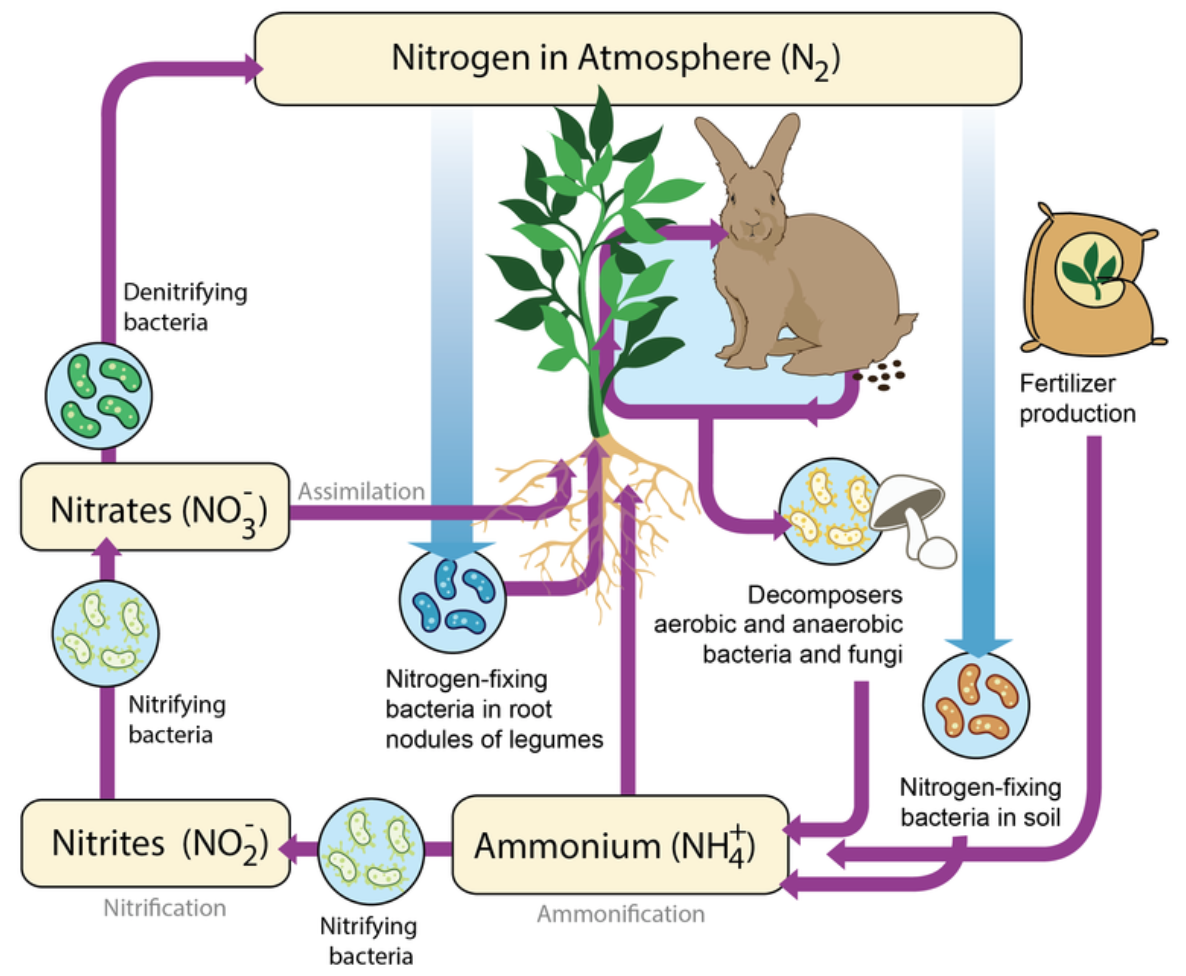
Plants cannot use nitrogen gas in the air to make organic compounds for themselves and the organisms that consume them. However, they can use nitrogen in the form of compounds such as nitrates, which they can absorb through their roots. The process of changing nitrogen gas to nitrates is called nitrogen fixation. It is carried out by bacteria, called nitrogen-fixing bacteria, that live in soil or on the roots of legumes such as peas. Nitrogen fixation is the primary source of nitrogen used by plants in most ecosystems.
When plants and other organisms die or release wastes, decomposers break down their organic compounds. In the process, they release nitrogen in the form of ammonium ions into the soil. The ammonium ions can be absorbed by plant roots. The ions can also be changed to nitrates by soil bacteria called nitrifying bacteria.
Not all of the nitrates produced by nitrogen-fixing and nitrifying bacteria are used by plants. Some of the nitrates are changed back to nitrogen gas by soil bacteria called denitrifying bacteria. This nitrogen returns to the atmosphere, thus completing the cycle.
Review
- What are biogeochemical cycles? Give examples of matter that have such cycles.
- Compare and contrast exchange pools and reservoirs in biogeochemical cycles. Give an example of each.
- How does water change as it moves through the water cycle? Illustrate your answer with examples.
- Most of Earth’s water is salt water in the ocean, and most precipitation falls back into the ocean. How is Earth’s supply of freshwater continuously renewed?
- Describe what may happen to liquid precipitation that falls on land.
- What is an aquifer? How can people access the water in an aquifer?
- Name two reservoirs of frozen water.
- How does the carbon cycle between organisms and the environment?
- Explain how human actions can change the carbon cycle.
- Identify some of the abiotic processes that cycle carbon slowly.
- How do living things obtain nitrogen from the environment?
- How is nitrogen returned to the atmosphere to complete the nitrogen cycle?
- True or False. Plants are involved in the water cycle.
- True or False. Most precipitation falls into the ocean because ocean water covers much of Earth’s surface.
Explore More
Attributions
- Water by drfuenteshernandez via Pixabay license
- Water cycle by Mariana Ruiz Villarreal (LadyofHats) for CK-12 Foundation, CC BY-NC 3.0
- Carbon Cycle by Mariana Ruiz Villarreal (LadyofHats) for CK-12 Foundation, CC BY-NC 3.0
- Mount St. Helens eruption by Austin Post, Public domain, via Wikimedia Commons
- Nitrogen cycle by Mariana Ruiz Villarreal (LadyofHats) for CK-12 Foundation, CC BY-NC 3.0
- Text adapted from Human Biology by CK-12 licensed CC BY-NC 3.0


