16.4: Consequences of Fossil Fuels
- Page ID
- 31659
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Benefits of Using Fossil Fuels
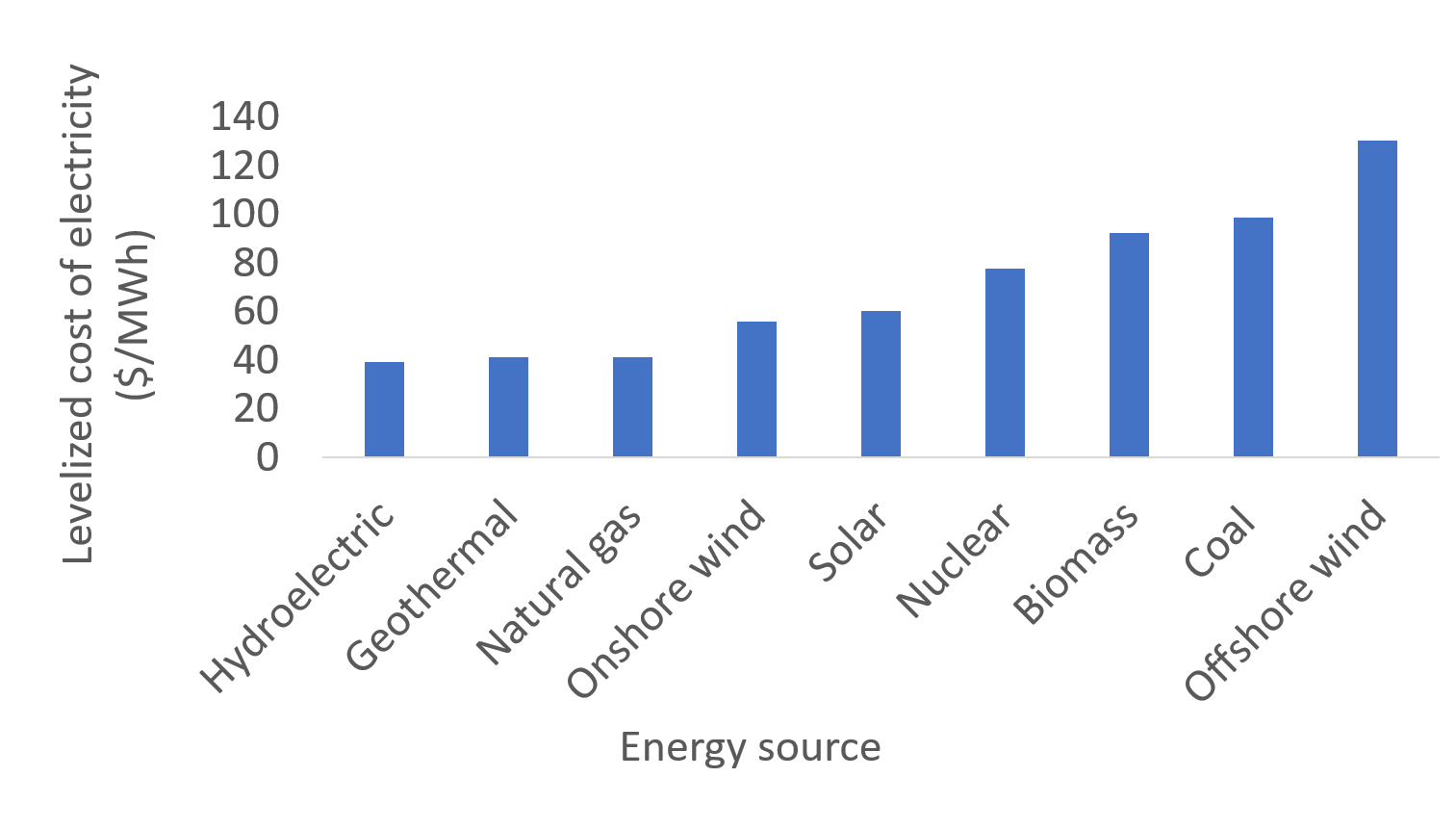
The world is heavily dependent on fossil fuels, and existing infrastructure and technologies facilitate their continued use. An advantage of using coal for electricity is that it plentiful and inexpensive, especially in the United States, which has larger coal reserves than any other country. Furthermore, coal mining is a source of jobs and tax income. Coal's economic advantage is dwindling, however, as technologies associated with renewable sources of energy, such as solar and wind, become more efficient and inexpensive. The U.S. Energy Information Administration compared the levelized cost of electricity (LCOE) for technologies that will begin use in 2023, and the cost of coal-generated electricity exceeded that of many renewable sources (figure \(\PageIndex{a}\)). The LCOE accounts for the building and operation costs of power plants, solar panels, wind turbines, etc.
Oil and natural gas continue to meet global energy needs. Despite expansions in renewable energy use, no alternative energy source is currently sufficient to replace oil and natural gas. (A combination of different renewable sources could be possible in the future.) While the United States does rely on imported oil, it continues to produce some oil and natural gas (mostly through fracking), bolstering U.S. energy independence. Local and state economics in regions rich in oil and natural reserves depend on continued extraction of these fossil fuels.
While all fossil fuels harm cause some degree of environmental harm, natural gas is a preferred fossil fuel for electricity generation when considering its environmental impacts. When burned, coal emits nearly double the carbon dioxide that natural gas does. Additionally, much less nitrogen oxides and sulfur dioxide (both air pollutants) are emitted from burning natural gas. It also does not produce ash as coal does (see below).
Health and Environmental Impacts
The negative impacts of fossil fuel use begin with the extraction of the resource. Fossils fuels are often located far from where they are utilized so they need to be transported by pipeline, tankers, rail or trucks. These all present the potential for accidents, leakage, and spills. Additional negative impacts are associated with processing, electricity generation, and disposal of the waste generated.
Coal Mining and Usage
Surface mining of coal disrupts local ecosystems above coal deposits as overburden is removed to access them (figure \(\PageIndex{b}\)). In mountaintop removal, a large volume of overburden is dumped over nearby habitats, causing further destruction (figure \(\PageIndex{c}\)). Mountaintop removal has affected large areas of the Appalachian Mountains in West Virginia and Kentucky. Habitat loss from coal mining decreases biodiversity, resulting in a loss of ecosystem services. Both surface and subsurface mining expose rocks that can contain contaminants, such as heavy metals or sulfates, which them leach into streams or other bodies of water. This not only harms aquatic life, but it also disrupts nutrient cycling. One of the largest environmental impacts of subsurface mining may be the methane gas that must be vented out of mines to make the mines a safe place to work. Methane gas is a potent greenhouse gas and contributes to climate change. Finally, the process of mining ultimately compacts soil. This combined with the loss of trees, which slow the flow of run-off and promote infiltration, increases the risk of flooding.


Coal miners face health hazards such as explosions, mine collapse, and exposure to toxic fumes. Black lung disease is a respiratory condition characterized by coughing and shortness of breath that occurs in miners exposed to too much coal dust. Residents near mines also risk exposure to coal dust and underground toxins following explosions. As a result of exposure to toxins, birth defects and other health problems are common in residents near mines.
Coal is considered the “dirtiest” source of energy because its combustion results in the most air pollution. Coal power plants emits a variety of air pollutants including sulfur dioxide, nitrogen oxide, and heavy metals. Sulfur dioxide and nitrogen oxide are sources of acid rain (acid deposition), smog, and health issues. Heavy metals cause neurological and developmental problems in humans and other animals. Burning of coal emits particulate matter and higher amounts of carbon dioxide per unit of energy than the use of oil or natural gas. Carbon dioxide is the most frequently emitted greenhouse gas and causes climate change. In 2018, electricity generation was responsible 27% of greenhouse gas emissions in the United States, and much of this was emitted from coal power plants. The transportation of coal usually relies on fossil fuels, releasing further pollution.
Ash (including fly ash and bottom ash) is a residue created when coal is burned at power plants. In the past, fly ash was released into the air through the smokestack, where it would contribute to particulate matter air pollution. Laws now require that much of the fly ash now must be be captured by pollution control devices, like scrubbers. In the United States, fly ash is generally stored at coal power plants or placed in landfills. Ash from storage or landfills can spill or leach into groundwater, resulting in water pollution.
Conventional Extraction Oil and Natural Gas
Exploring and drilling for oil degrades land and ocean habitats. On land, extensive infrastructure such as road networks, transport pipelines and housing for workers are needed to support a full-scale drilling operation. These can pollute soil and water, fragment habitats, and disturb wildlife. Extraction of oil and natural gas is also hazardous for workers, who have a high incidence of cancer and heart disease.
Human-caused oil spills in rivers and oceans harm ecosystems. From an economic perspective, oil spills disrupt the fishing industry and tourism. Oil spills at sea are generally much more damaging than those on land, since they can spread for hundreds of nautical miles in a thin oil slick which can cover beaches with a thin coating of oil. This can kill sea birds, mammals, shellfish and other organisms it coats. Oil spills on land are more readily containable if a makeshift earth dam can be rapidly bulldozed around the spill site before most of the oil escapes, and land animals can avoid the oil more easily.
Oil spills can result from supertanker accidents such as the Exxon Valdez in 1989, which spilled 10 million gallons of oil into the rich ecosystem of coastal Alaska and killed massive numbers of animals. The largest marine oil spill began in April 2010 when a natural gas explosion at an oil well 65 km offshore of Louisiana occurred on Deepwater Horizon Oil Rig. It killed 11 employees and flowed for 3 months in 2010, releasing an estimated 200 million gallons of oil (figure \(\PageIndex{d}\)). Wildlife, ecosystems, and people’s livelihood were adversely affected. A lot of money and huge amounts of energy were expended on immediate clean-up efforts. The long-term impacts are still not known. The National Commission on the Deepwater Horizon Oil Spill and Offshore Drilling was set up to study what went wrong. The worst oil spill ever occurred during the Persian Gulf war of 1991, when Iraq deliberately dumped approximately 200 million gallons of oil in offshore Kuwait and set more than 700 oil well fires that released enormous clouds of smoke and acid rain for over nine months.

During an oil spill on water, oil floats to the surface because it is less dense than water, and the lightest hydrocarbons evaporate, decreasing the size of the spill but polluting the air. Then, bacteria begin to decompose the remaining oil, in a process that can take many years. After several months only about 15% of the original volume may remain, but it is in thick asphalt lumps, a form that is particularly harmful to birds, fish, and shellfish. Cleanup operations can include a variety of components, but each has its procs and cons. Skimmer ships that vacuum oil from the water surface, but these are effective only for small spills. Controlled burning works only in early stages before the light, ignitable part evaporates, but this also pollutes the air. Dispersants are detergents that break up oil to accelerate its decomposition, but some dispersants may be toxic to the ecosystem. Bioremediation refers to adding microorganisms that specialize in quickly decomposing oil, but this can disrupt the natural ecosystem.
Unconventional Extraction of Oil and Natural Gas
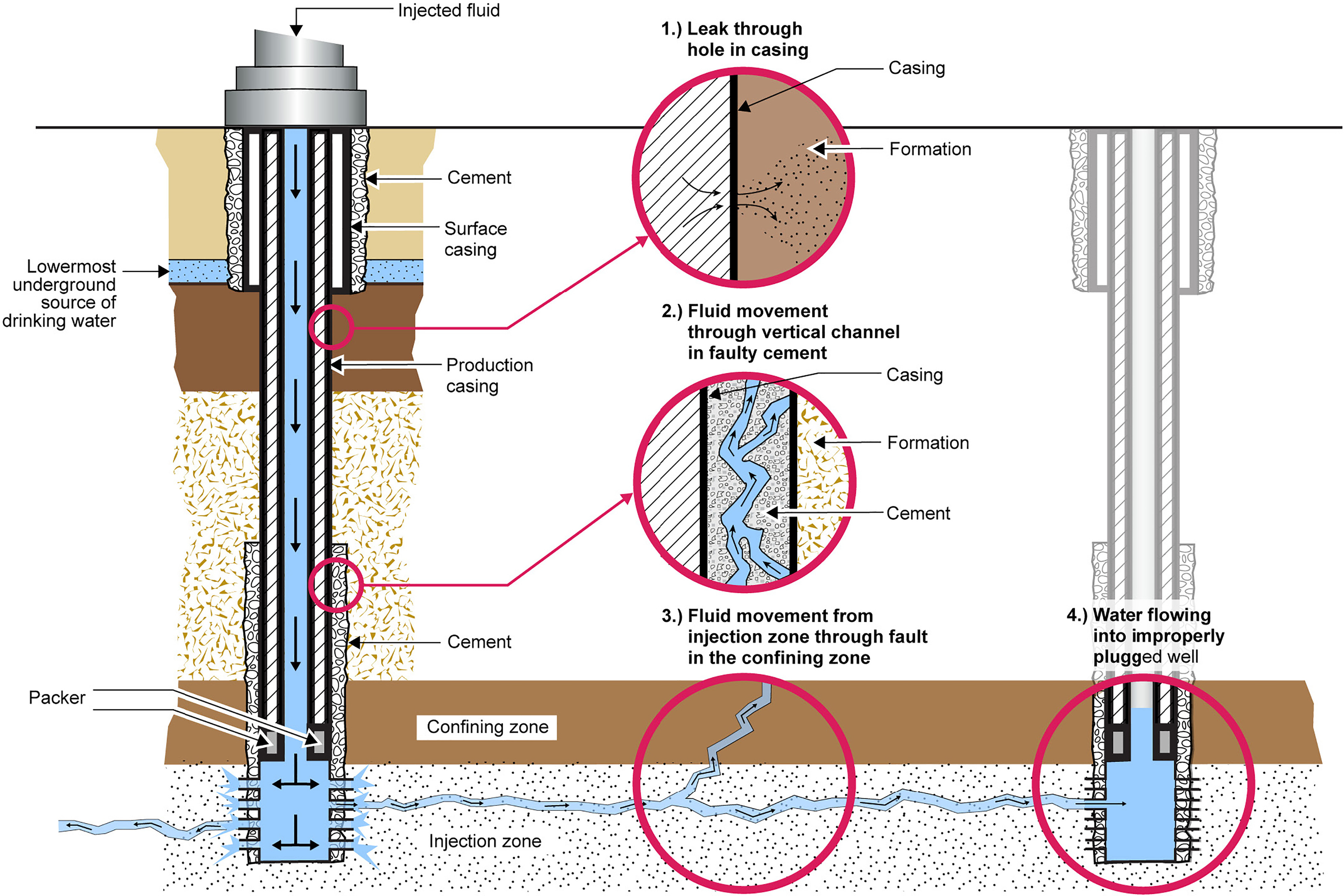
Fracking causes more environmental damage than conventional extraction. The considerable use of water (figure \(\PageIndex{e}\)) may affect the availability of water for other uses in some regions, and this can affect aquatic habitats. In fact, fracking consumes more water than the use of nuclear energy, coal, or conventional oil and natural gas. If mismanaged, hydraulic fracturing fluid can be released by spills, leaks, or various other exposure pathways that contaminate land and groundwater (figure \(\PageIndex{f}\)). Fracking fluid flowback – the fluid pumped out of the well and separated from oil and gas – not only contains the chemical additives used in the drilling process but also contains heavy metals, radioactive materials (which release radiation), volatile organic compounds, benzene (a carcinogen), toluene, ethylbenze, xylene, and other toxic air pollutants. Volatile organic compounds (VOCs) can react with the atmosphere to form ground-level ozone, which is associated with respiratory disease. Toulene can cause dizziness, confusion, headaches, and miscarriages. Ethylbenzene is a possible carcinogen that also causes dizziness, eye irritation, and hearing loss. Xylene also causes dizziness and headaches and furthermore can be fatal at high concentrations. In some cases, this contaminated water is sent to water treatment plants that are not equipped to deal with some of these classes of contamination. Finally, injecting wastewater for disposal can even induce earthquakes.
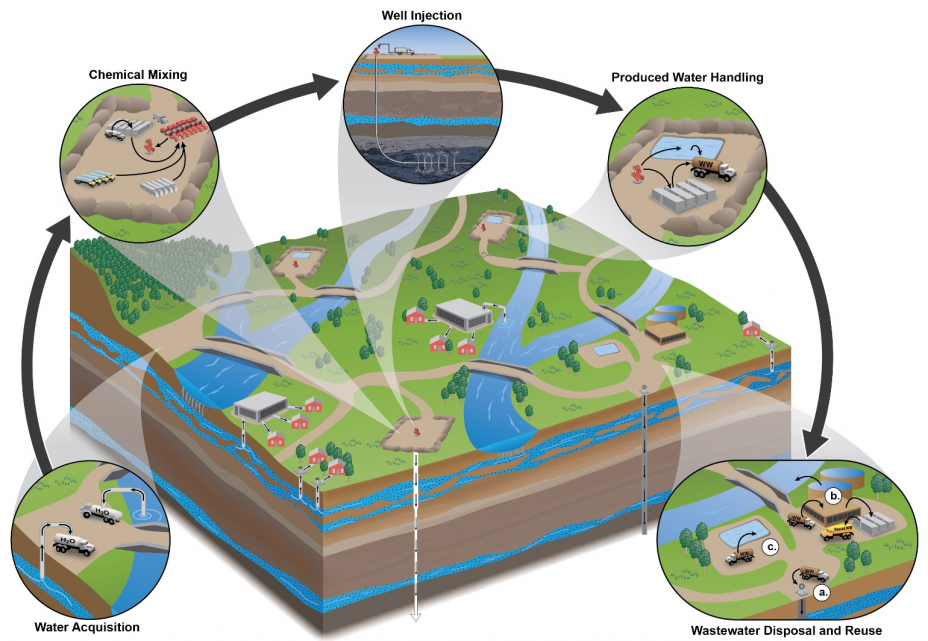
Other unconventional sources of fossil fuels can also harm the environment. Surface mining of tar sands or oil shales requires the removal of all vegetation and leaves pollutants behind, causing habitat loss (figure \(\PageIndex{g}\)).

Transportation, Refineries, and Combustion
Natural gas is released into the atmosphere from coal mines, oil and gas wells, and natural gas storage tanks, pipelines, and processing plants. These leaks are the source of about 25% of total U.S. methane emissions, which translates to three percent of total U.S. greenhouse gas emissions, contributing to climate change. When natural gas is produced but cannot be captured and transported economically, it is “flared,” or burned at well sites, which converts it to carbon dioxide. This is considered to be safer and better than releasing methane into the atmosphere because carbon dioxide is a less potent greenhouse gas than methane. However, when natural gas with high concentrations of the toxic gas hydrogen sulfide is flared, it produces carbon dioxide, carbon monoxide, sulfur dioxide, nitrogen oxides, and many other compounds (see Air Pollution for more details).
Leaks also happen when we use petrochemicals on land. For example, gasoline sometimes drips onto the ground when people are filling their gas tanks, when motor oil gets thrown away after an oil change, or when fuel escapes from a leaky storage tank. When it rains, the spilled petrochemicals get washed into the gutter and eventually flow to rivers and into the ocean. Another way that oil sometimes gets into water is when fuel is leaked from motorboats and jet skis. When a leak in a storage tank or pipeline occurs, petrochemicals can also get into the ground, and the ground must be cleaned up. To prevent leaks from underground storage tanks, all buried tanks are supposed to be replaced by tanks with a double lining.
Oil refining emits a variety of toxins and is the single largest source of benzene (figure \(\PageIndex{g}\)). As a result, residents living near oil refineries have a high incidence of cancer, asthma, and birth defects. When petrochemicals such as gasoline or diesel are burned, they release a variety of air pollutants, including carbon dioxide (a cause of climate change), sulfur dioxide, nitrous oxides, volatile organic compounds (VOCs), particulate matter, and lead (see Air Pollution for more details). The transport of oil by ship or trunk also requires energy in the form of fossil fuels, generating more pollutants. Compared to oil and coal, burning natural gas releases the fewest pollutants and greenhouse gases.

Solutions
Reclamation can mitigate the habitat damage that results from mining or extracting fossil fuels. It involves restoring the land to an extent after mining or extraction is complete. This can entail returning displaced land and covering with top soil, which protects organisms from heavy metals, radioactive materials, and other underground toxins. Additionally, acids, which often form from the leaching of sulfates from underground rocks, may be neutralized. Vegetation is then planted, and water flow if disrupted is somewhat restored. Of course, the intricate topography, network of streams, and mature vegetation (such as large trees in forest) that may have been present prior to mining cannot be recreated, but reclamation makes it easier for native species to begin recolonizing the area.
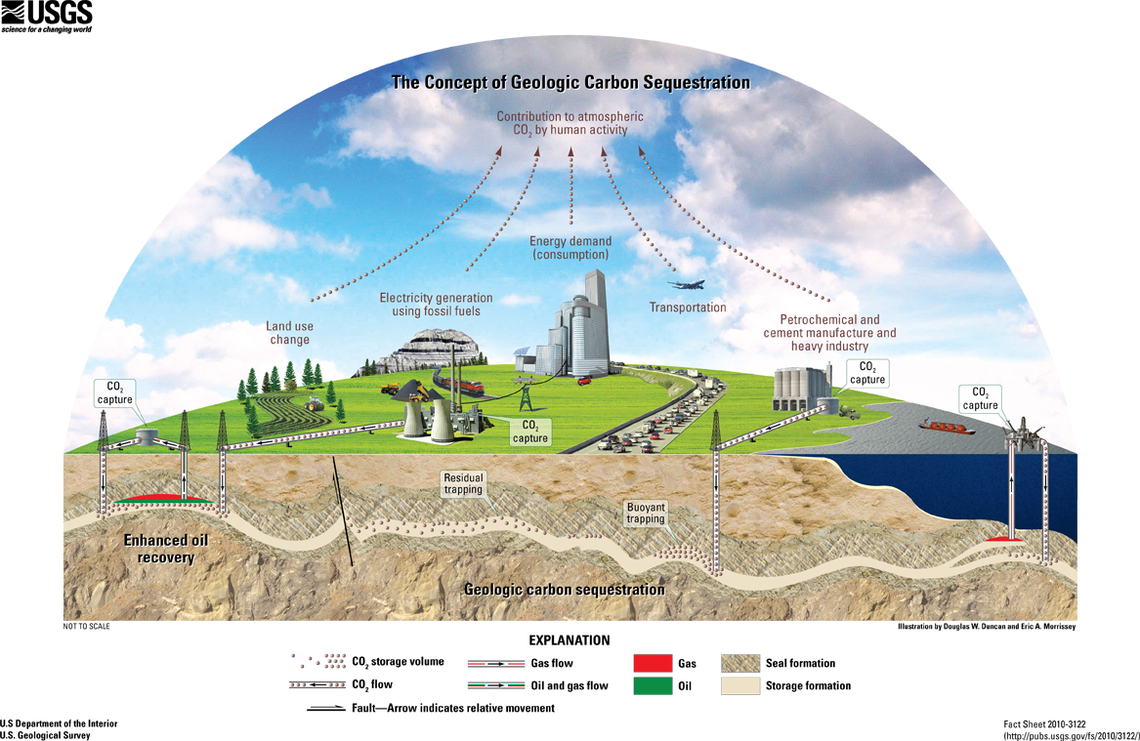
Clean coal technologies can limit the air pollution released when burning coal. Some of these technologies remove toxins from coal before burning it while others capture toxins that are released while burning coal. For example, smokestack scrubbers in power plants clean sulfur dioxide, nitrous oxide, particulate matter, and mercury from the smoke before it is released. Carbon capture and sequestration involves capturing carbon dioxide released and storing it, but it requires 25-40% more energy, reducing the efficiency of coal (figure \(\PageIndex{h}\)). In this process, smoke from a coal power plant is passed through a solvent to trap carbon dioxide, but other waste gases are still released in the smoke. Carbon dioxide is then separated from the solvent. Some can be used in industry (such as for carbonated beverages or to tertiary recovery of oil), and the rest is sequestered (stored) underground. Note that clean coal technologies can reduce coal's contribution to climate change and reduce the amount of toxins that are released, but it does not fully prevent coal-generated air pollution (figure \(\PageIndex{i}\)).
Because fossil fuels are nonrenewable, reserves will eventually be depleted, and the world will need to fully rely on other energy sources. Those concerned about the environmental and health consequences of fossil fuels advocate for making this transition as soon as possible. This is because the technologies and practices discussed above do not fully prevent fossil fuels from causing environmental damage and causing health hazards for workers and the general public. The next two chapters discuss nuclear energy and renewable energy, which are alternatives to fossil fuels. As even these alternatives have their disadvantages, energy conservation (using energy more efficiently and limiting unnecessary energy use) is also critical.
Attribution
Modified by Melissa Ha from the following sources:
- Challenges and Impacts of Energy Use, Non-Renewable Energy Sources, and Water Pollution from Environmental Biology by Matthew R. Fisher (licensed under CC-BY)
- Fossil Fuels from An Introduction to Geology by Johnson et al. (licensed under CC-BY-NC-SA)
- Chapter 4: Non-Renewable Energy from Introduction to Environmental Science: 2nd Edition (2018) Biological Sciences Open Textbooks by Zehnder, Caralyn; Manoylov, Kalina; Mutiti, Samuel; Mutiti, Christine; VandeVoort, Allison; and Bennett, Donna (licensed under CC-BY-NC-SA).


