Voltage-Gated Sodium Channel
( \newcommand{\kernel}{\mathrm{null}\,}\)
Written by Henry Jakubowski, Emily Schmitt Lavin, Arthur Sikora, Subhasish Chatterjee, and Kristen Procko
| Literature-Based Guided Assessment (LGA) | Voltage-Gated Sodium Channel |
Introduction
Eukaryotic voltage-gated sodium (NaV) channels generate and sustain action potentials in nerve and muscle cells by moving Na+ ions from the outside to the inside of the cell. This increases and makes positive the transmembrane potential of the cell, which at rest is approximately -70 mV (more negative inside). Once activated, the channel undergoes a fast inactivation (1-2 ms), without which the firing of nerves and muscles becomes dysregulated, a potentially lethal effect. Please view the information in Chapter 11.3 on the voltage-gated sodium channel before you do these guided assessment activities.
The questions below are derived from a paper from Jiang et al. on the structure and properties of the α-scorpion toxin LqhIII (MW 7,000) bound to rat cardiac sodium channel NaV1.5 (MW 227,000) by. (Jiang, D., Tonggu, L., Gamal El-Din, T.M. et al. Structural basis for voltage-sensor trapping of the cardiac sodium channel by a deathstalker scorpion toxin. Nat Commun 12, 128 (2021). https://doi.org/10.1038/s41467-020-20078-3. Creative Commons Attribution 4.0 International License. http://creativecommons.org/licenses/by/4.0/)
The Lqh toxin is made by Leiurus quinquestriatus hebraeus. It is found in North Africa, the Middle East, and Western India and is shown below.
htt.://en.wikipedia.org/wiki/Deathstalker.
The study shows how the deathstalker scorpion (LqhIII) toxin inhibits the fast inactivation of cardiac sodium channels (Nav1.5). In other words, you will see how the toxin keeps the channel open longer than it would be open in its absence.
- In the absence of toxin, the sodium channel NaV1.5 returns in 1-2 ms to an inactive state when an 'inactivation gate" moves to occlude the open pore.
- The α-scorpion toxin LqhIII inhibits the return of the channel to the inactive state. Since the toxin inhibits the channel's fast inactivation of Na+ ion flow into the cell, the channel stays open longer.
The toxin leads to the inhibition of the normal fast inhibition (inactivation) of the channel. Hence the channel stays open (activated) longer. This is analogous to the statement that the enemy of my enemy is my friend!
Here are some links within Fundamentals of Biochemistry that give background information about channels and techniques used in the article:
- Diffusion Across a Membrane - Channels
- Voltage-gated ion channels
- Potassium ion channels
- Sodium ion channels
- Molecular Dynamics Simulations
Techniques to study the Na Channel
For the experiments described in the paper, the rat sodium cardiac channel NaV1.5 was purified, its structure determined, and its functional properties (the regulated movement of Na+ to the inside of the cell - electrophysiology) in human epithelial cells measured. The protein is expressed in rat cardiac cells and is found in the cell membrane of the cell. The iCn3D image below shows the alpha chain of rat cardiac NaV1.5 (6UZ3) embedded in a simple bilayer (DMPC) to model how it might appear in a cardiac cell membrane bilayer.
PDB coordinates based on S. Jo, T. Kim, V.G. Iyer, and W. Im (2008). CHARMM-GUI: A Web-based Graphical User Interface for CHARMM. J. Comput. Chem. 29:1859-1865. S. Jo, T. Kim, and W. Im (2007) Automated Builder and Database of Protein/Membrane Complexes for Molecular Dynamics Simulations. PLoS ONE 2(9):e880
Complete the flow chart below to show two different approaches that could be used to purify the protein and prepare it for structural and functional studies. On the left show steps you could use to directly purify the protein from rat hearts. On the right use the rat gene (Scn5a) for the channel as the starting point for purification. Here are some links for review if needed.
- Answer
-
From heart tissue:
From heart tissue DNA
Here are some review links:
Key components of the buffer solution used to purify the channel include HEPES and 1% (w/v) n-dodecyl-β-D-maltopyranoside. Their structures are shown below. Describe the role of each.
- Answer
-
.
The following iCn3D shows the interaction of the purified sodium channel with n-dodecyl-β-D-maltopyranoside (BDDM). Explain the differences between the iCn3D models showing the protein in a bilayer and interacting with the BDDM.
- Answer
-
.
The elution of the complex on a size exclusion column (Panel A) and the analyses for the eluted fractions by SDS-PAGE (Panel B) are shown in the figure below. The α-scorpion toxin LqhIII: rat cardiac sodium channel NaV1.5 complex elutes in the area of the first peak shown in blue.
a. How do molecules separate on size exclusion chromatography?
b. Compare the molecular weights of the first peak to the second complex peak in Panel A.
c. Which band(s) in Panel B likely represent the rat cardiac sodium channel NaV1.5 based on the intensity of the stained band? The toxin LqhIII is the lowest band. ( bands at 17.5K and 12.5 are FGF12b and calmodulin, respectively, which were added to stabilize the channel)
d. Why do the channel and toxin elute together in the size exclusion column shown in Panel A, but are separate bands in PAGE gel in Panel B?
e. How could you get the complex to separate as two peaks, the free NaV1.5 channel, and the free α-scorpion toxin LqhIII?
f. To get information on the receptor, go to Uniprot and paste in rat cardiac sodium channel NaV1.5 into the search box. Go to Sequence and Isoform in the left panel and find the actual MW. Knowing this, what are the major bands at about 170K and 60K?
Figure: Purification of the recombinant NaV1.5C/LqhIII complex. a. Representative size-exclusion chromatography profile of purified rNav1.5C/LqhIII. Peak fractions collected for cryo-EM grid preparation are shown in blue. b. SDS-PAGE of the size exclusion peak fractions stained by Coomassie blue.
- Answer
-
.
CryoEM was used to determine the structure of the sodium channel:toxin complex. Here is a short YouTube video that describes the technique. Also review the appropriate part of Chapter 3.3: Analyses and structural predictions of protein structure.
Describe the temperature conditions for protein samples in cryoEM. What is the reported resolution of cryo EM structure? How does this compare to X-ray structures? What are some advantages of using cryoEM over X-ray crystallography and NMR to determine the structure of proteins?
- Answer
-
.
Molecular dynamics was also used to probe the conformational changes in the structure of the complex on the picosecond (10-12 s) to nanosecond (10-9 s) time scale. For a review of molecular dynamics, see Chapter 3.3: Analyses and structural predictions of protein structure. It can be used to probe dynamic changes in protein structure which cryoEM can't.
Answers these multiple choice questions (created by AIPDF through ChatGPT4 -paid version using this prompt: Write 5 question for a biochemistry major about the use of molecular dynamics and the finding in the paper)
1. What was one of the primary uses of molecular dynamics in this research?
- A) Predicting the behavior of NaV channels without toxins.
- B) Analyzing hydration and Na+ permeation through the rNaV1.5C/LqhIII complex.
- C) Studying the interaction between different toxins.
- D) Predicting the behavior of potassium channels.
2. In the molecular dynamics simulation analysis, what was aligned to the initial position for each snapshot?
- A) The α-toxin LqhIII.
- B) The voltage-sensing domain IV.
- C) The Cα atoms from pore transmembrane helices.
- D) The fast inactivation gate.
3. Approximately how long were the unrestrained "production" simulations generated?
- A) 10.35 ns.
- B) 5000 steps.
- C) 300 ns.
- D) 2 fs.
4. Based on the molecular dynamics analyses, what was observed about the activation gate structure of the rNaV1.5C/LqhIII complex?
- A) It was fully open for Na+ conductance.
- B) It was functionally closed for Na+ conductance.
- C) It was in a metastable state.
- D) It showed no significant change from the rNaV1.5C structure.
- Answer
-
.
The authors used two types of electrophysiological techniques, patch clamp, and voltage clamp. Here is some brief background.
In a whole-cell patch clamp experiment, a pipet is placed on a cell, and suction is applied until a tight seal, indicated by a sharp rise in electrical resistance (gigaohm level) is made. This is illustrated in the figure below.
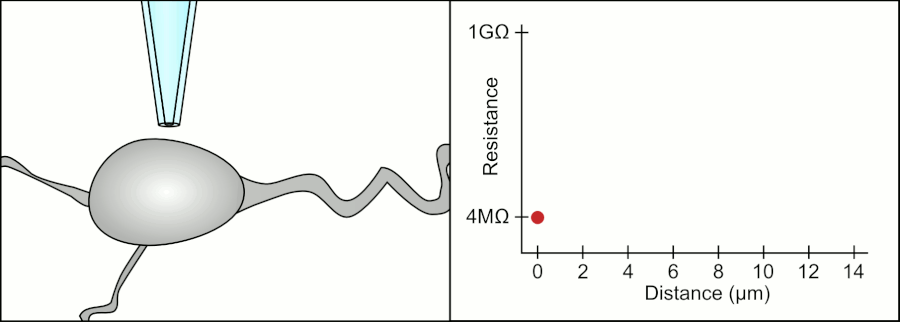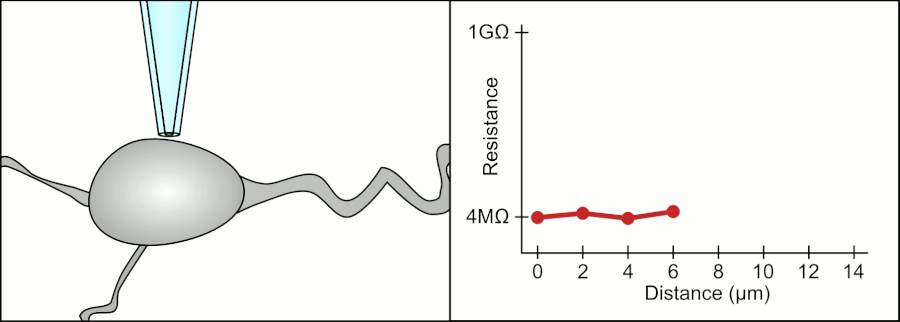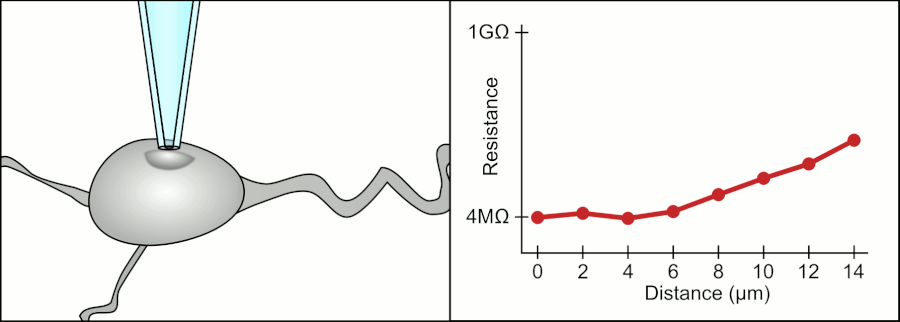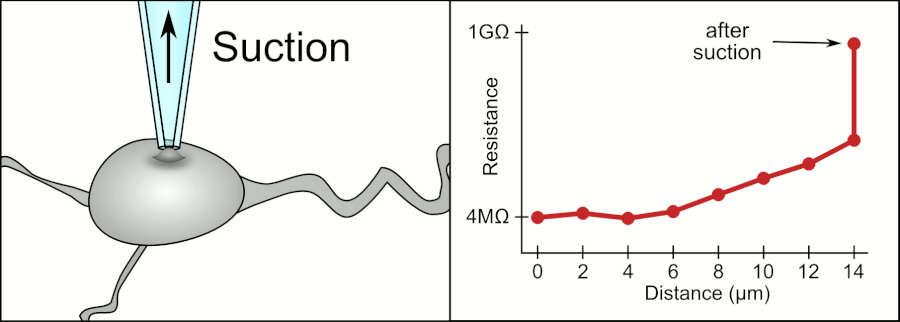
Patch Clamp Resistance. Formation of gigaseal. Holst. https://en.wikipedia.org/wiki/Automa..._Animation.gif. CC BY-SA 3.0
The cell can then be connected to a patch clamp chip in such a way that transmembrane potential or current can be measured on single-channel ion flow. This is illustrated in the figure below.
Holst. Patch Clamp Chip. Batch clamp chip showing a gigaseal, whole-cell recording configuration, and the ion channel and whole cell current. https://en.wikipedia.org/wiki/Automated_patch_clamp#/media/File:Patch_Clamp_Chip.svg. CC BY-SA 3.0
In patch-clamp fluorometry, part of the cell membrane is sucked into the tip with the seal intact. Fluorescent ligands can be applied to one side of the membrane that contains an ion channel and current measurements were made as illustrated in the figure below. Alternatively, as in this paper, side chains in the S4 voltage sensor were labeled with a fluorophore, and changes in fluorescence were observed with changes in membrane potential.

Patch-Clamp Fluorometry. https://www.uniklinikum-jena.de/phys...n/Methods.html
Answers these general multiple-choice questions (created by AIPDF through ChatGPT4 -paid version using this prompt: Write five multiple-choice questions about the use of patch clamp techniques to measure sodium currents in cells)
1. What is the primary purpose of the patch-clamp technique in cellular electrophysiology?
- A) To visualize cell structures.
- B) To measure the concentration of sodium ions inside cells.
- C) To record ion currents across cell membranes.
- D) To stimulate cellular growth.
3. In a typical neuron at resting potential (-70 mV) and in this study (epithelial cells transformed with the rat channel, what is the direction of the sodium current when sodium channels open?
- A) Inward, into the cell.
- B) Outward, out of the cell.
- C) There is no movement of sodium.
- D) Both inward and outward simultaneously.
4. Which of the following factors can influence the magnitude and direction of sodium currents measured using patch-clamp techniques?
- A) The concentration of potassium ions outside the cell.
- B) The voltage across the cell membrane.
- C) The pH of the cell cytoplasm.
- D) The size of the cell.
5. Why might a researcher use drugs or toxins during a patch-clamp experiment measuring sodium currents?
- A) To increase the size of the cell.
- B) To modulate or block sodium channels and observe the effects.
- C) To change the color of the cell.
- D) To stimulate cell division.
- Answer
-
.
Answer these general multiple-choice questions about patch-clamp fluorometry. (created by AIPDF through ChatGPT4 -paid version using this prompt: write 5 multiple choice questions of patch clamp fluorometry in which key amino acids in a membrane protein are labeled with a fluorophore)
1. What is the primary advantage of combining patch-clamp with fluorometry in studying membrane proteins?
- A) It allows simultaneous measurement of electrical activity and conformational changes.
- B) It increases the fluorescence of all amino acids.
- C) It enhances the electrical activity of the protein.
- D) It allows visualization of the entire cell in detail.
2. Why are specific amino acids in a membrane protein labeled with a fluorophore in patch-clamp fluorometry?
- A) To increase the size of the protein.
- B) To change the electrical properties of the protein.
- C) To detect specific conformational changes in the protein during activity.
- D) To make the protein more soluble in water.
3. Which property of the fluorophore is crucial for patch-clamp fluorometry?
- A) Its electrical charge.
- B) Its sensitivity to changes in the local environment or protein conformation.
- C) Its ability to increase protein activity.
- D) Its color in visible light.
4. In which scenario would patch-clamp fluorometry be especially useful?
- A) When studying the overall shape of a cell.
- B) When investigating the relationship between ion channel gating and conformational changes.
- C) When trying to increase the fluorescence of a solution.
- D) When observing the movement of proteins inside the cell.
5. What is a critical consideration when choosing a fluorophore for labeling amino acids in patch-clamp fluorometry?
- A) The taste of the fluorophore.
- B) The electrical conductivity of the fluorophore.
- C) The photostability and brightness of the fluorophore.
- D) The size of the fluorophore molecule.
- Answer
-
.
Nonstructural Lab Studies of LqhIII Toxin Effects on Rat Sodium Channel NaV1.5 (rNaV1.5C)
HEK293S GnTI– (epithelial-like) cells were transformed with the rat cardiac sodium channel NaV1.5 (rNaV1.5C). The cells were then studied in the absence and presence of the toxin at varying times after toxin addition and at various concentrations of the toxin. The opening and closing of the channel were determined by measuring changes in the Na+ currents into the cell on channel opening.
The resting potential of a cell is around -70 mV (more negative inside). When the transmembrane potential is depolarized by raising the transmembrane potential to around -55 mV or even more positive, the Na+ channels are activated, and an inward Na+ current (black line in a modified form of Figure 1a from the paper below) which goes downward by convention) through the channel occurs. This is followed by a quick inactivation of the channel and the return to the baseline flow of ions. In the experiment below, the potential was raised from -100 mV (channel closed) to 0 mV (channel open). What is happening to the NaV1.5 Na+ channel during these 10 ms? What is special about the current at 6 ms (indicated by the dashed vertical line)
- Answer
-
.
Figure 1a from the paper (modified) below shows a series of lines of different colored (black to red) representing Na+ currents obtained at 0 (black line) and increasing concentrations (gray through red) of the LqhIII scorpion toxin. Let's assume that the downward Ipeak =1. The values of I 6ms/Ipeak, calculated from the approximate values shown on the graph, are also shown on the vertical axis Does the toxin alter the immediate response of the cells after the channel was activated? What effect does increasing [toxin] have on the response of the cell? Offer a structural explanation of how the toxin affects the cell by suggesting changes in the toxin-bound structure.
- Answer
-
.
Figure 1a (left) from the paper below shows the dependency of the inhibition of the quick inactivation of the channel on the log of the LqhIII concentration. When the transmembrane potential is set to 0 mV (as in this experiment), the channel should open and the current would be maximal. The data points in the graph are close to the ones estimated in the graph from the previous question.
a. In the absence of the toxin, what should the current I be at 6 ms compared to the maximal Na+ current? That is, what would be the value of I6ms/Ipeak?
b. Is the channel completely inactivated in the presence of the toxin?
c. At 6 ms, what concentration of toxin (nM) causes 50% inhibition of the maximum effect of the inhibitor on the normal rapid inactivation of the channel?
- Answer
-
.
In the next experiment, cells were kept at -120 mV at one fixed concentration (100 nM) of toxin. The toxin was left to incubate with the cells for various times up to 20 min. After the incubation time, the transmembrane potential was changed to 0 mV to activate the channel, and inward Na+ currents were measured. The results are shown in the top inset graph in Figure 1b from the paper. (Note: It is unclear from the paper if the control was determined at 0 min with 100 nM toxin or no toxin.)
a. Did the length of time cells were pre-incubated with the 100 nM toxin affect Na+ currents after depolarization of the cells? How did the effects on the cells depend on the preincubation time?
b. Describe and explain these results
- Answer
-
.
Here is another interesting feature of toxin binding. The toxin binds to a site on the resting state of the NaV1.5C with high affinity. When the cell becomes depolarized (made more + inside the cell), the affinity for the toxin decreases so it starts to dissociate. The affinity of the toxin for NaV1.5C decreases with increasing + transmembrane potential. At very high positive potentials (+100 mV) it appears not to bind.
What might account for the decreasing affinity of the bound toxin for the NaV1.5C with an increasing transmembrane potential?
- Answer
-
.
Time course experiments were conducted on the complex at 100 nM of LqhIII scorpion toxin. A three-pulse protocol can be applied to alter membrane potentials:
- 1st: a pulse from −120 mV to +100 mV for the indicated times then
- 2nd: a 50-ms hyperpolarizing pulse (make membrane potential very negative, perhaps around -100 mV)
- 3rd: a pulse of 50 ms to 0 mV
Note that steps 2 and 3 both occur with 0.1 s. What is the purpose of each pulse?
- Answer
-
.
Figure 1c from the paper below shows results for a set of 3-pulse designed to allow recovery of the fast inactivation which was blocked by the previous toxin binding. Note that the transmembrane potential for the first pulse was +100 mV
a. Describe what happens to the channel and complex.
b. Explain the results
c. Summarize thermodynamic and kinetic features that make the toxin so effective.
- Answer
-
.
Structural Studies of LqhIII Toxin Effects on Rat Sodium Channel NaV1.5 (rNaV1.5C) - CryoEM
Before we discuss in detail the structure of the sodium channel and its complex with the toxin, let's look at an important attribute of molecules that helps determine their function, the actual size of the species involved.
The Na+ and the K+ voltage-gated ion channels must have an open pore when the channel protein is active (Na+ ions move across the channel). Extracellular Na+ and the K+ ions don't exist as "naked" ions but they are hydrated by water in an aqueous extracellular environment. The figure below shows the relative sizes of these Group I cations and their hydrated forms, in comparison to the diameter of the open NaV1.5 pore in the channel. Answer the following questions. The red sphere (c) represents the calculated value of the diameter of water assuming its volume when it is bound to a protein is 25 Å3.
1. Which represents the "naked" (nonhydrated) size of the K+ ion?
2. Which represents the hydrated Na+ ion?
3. Based on the pore size alone, which of these species could diffuse through the pore?
- Answer
-
.
The approximate relative sizes of the hydrated Na+ ion, pore opening, toxin, and the NaV1.5 protein are shown in the figure below along with the relative width of the bilayer (BL). A cardiac epithelium cell is shown as a rectangle to the right. A red dot  (not visible in the large figure) in the membrane surrounded by the red-dotted circle represents a single NaV1.5 channel.
(not visible in the large figure) in the membrane surrounded by the red-dotted circle represents a single NaV1.5 channel.
1. Which likely represents the pore?
2. Which represents NaV1.5 channel protein?
3. Which represents the toxin?
- Answer
-
.
If you hadn't read the paper, where would you consider the most likely location for a toxin to bind to affect the function of Nav1.5? Circle the most likely toxin binding site in the schematic below. Based on the paper, where did it bind? Redraw the toxin in the correct location based on the paper.
- Answer
-
.
How is this toxin (LqIII) different in terms of its binding location from other toxins that interfere with the functioning of Nav1.5? What is the effect of the toxin on the channel and the symptoms of this venom?
- Answer
-
.
What amino acids should be present in the S4 segment of Nav1.4 and why?
- Answer
-
.
The figure below shows an interactive iCn3D model of the rat sodium channel NaV1.5 bound to the LqhIII toxin (7k18).
Rat sodium channel NaV1.5 bound to the LqhIII toxin (7k18). (Copyright; author via source). Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...jpz8CFQajMKJe7
Domains I-IV are shown in gray, yellow, green, and cyan, respectively. The magenta parts are simply extensions or connectors of these domains. The LqhIII toxin is shown in dark blue. The Ile-Phe-Met (IFM) sequence of the inactivation gate, which is involved in closing the pore during fast inactivation, is depicted as black spheres and labeled.
It is essential to note that certain stretches of amino acids are missing from the structure. These include AAs 1-120 (N-terminus), 298-301, 425-501, 602-608, and 1781-1838.
Here is a link to an external iCn3D structure of NaV1.5 bound to the LqhIII toxin (7k18) that also highlights the DEKA (Asp, Glu, Lys, and Ala) side chains that compose the selectivity filter: https://www.ncbi.nlm.nih.gov/Structu...69c32c441ca0cd
a. To which domain does the LqhIII toxin bind?
b. Is the binding site close to the pore-forming segments of the domain or the voltage-sensitive segments?
c. Does the layer of red spheres represent the outer (extracellular) or inner (intracellular) leaflet of the membrane?
d. Offer reasons that parts of the protein are missing from the structure.
- Answer
-
.
The IFM motif has been shown to be conserved across all voltage-gated sodium channels.
a. What role does it play in these channels and in Nav1.5 and why?
b. The cause of Paroxysomal Extreme Pain Disorder (PEPD), an extremely rare disease with only 15 known affected families, appears to be mutations in the IFM motif which leads to increased sensations of pain. What is a likely effect of the mutations in the IFM motif?
https://en.wikipedia.org/wiki/Paroxy..._pain_disorder
- Answer
-
.
The figure below shows a different interactive iCn3D model of the rat sodium channel NaV1.5 bound to the LqhIII toxin (7k18) without a membrane representation for clarity. It shows the selectivity filter DEKA (spacefill, CPK colors), the inactivation gate IFM and the IFM "internal receptor" F1651, L1660, and N1662 (spacefill, CPK colors), and a ring of hydrophobic residues V413, L941, I1471, and I1773 (spacefill, black) that in the closed state completely seal off the cytoplasmic opening in the pore.
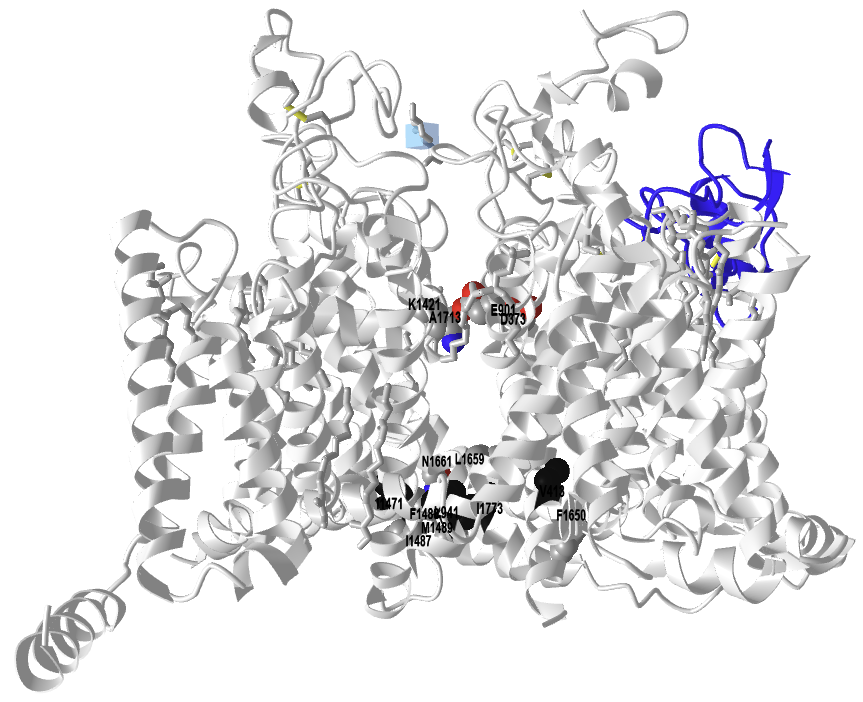.png?revision=1)
Rat sodium channel NaV1.5 bound to the LqhIII toxin without a membrane representation (7k18). (Copyright; author via source). Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...pPzakHu6Ew6WH9
After viewing the structure from all angles, do you think that the toxin:NaV1.5 complex looks closed, open, or inactivated form? Explain
- Answer
-
.
Structural Studies of LqhIII Toxin Effects on Rat Sodium Channel NaV1.5 (rNaV1.5C) - Molecular Dynamic Simulations
Here is a link to an introduction to Molecular Dynamics Simulations
Now let's look at some data to see if the pore is really open, partially open, or closed in the toxin:channel complex. One clue is if the structure shows water in the pore as the Na+ ions must be hydrated to pass through the pore (the opposite case is seen with K+ channels when K+ pass through stripped of water). Molecular dynamic (MD) simulations were done on the NaV1.5 with and without the toxin to simulate the environment in the channel opening. The results of the MD simulations are shown below in Figures 6 a and c from the paper.
Molecular dynamics analysis of hydration and Na+ permeation through the rNaV1.5C/LqhIII complex.
Panel a shows a side view of rNaV1.5C (orange ribbons; domains II and IV) from MD simulations highlighting Na+ ions (blue spheres), the water-occupied volume within a cylinder of radius 8.5 Å (red surface), and the protein-occupied volume within a cylinder of radius 12 Å (colorless surface). The cavity within the pore is outlined with a black rectangle. The region of the intracellular activation gate is shown as a purple band.
Panel c shows molecular representations of the gate containing Nwater = 3 (left) or 15 (right) water molecules
Based on these studies, do you believe the pore is closed, open, or partially open?
- Answer
-
.
Detailed Structural Analyses of LqhIII Toxin Effects on Rat Sodium Channel NaV1.5 (rNaV1.5C)
From the iCn3D model, write the sequence of the S4 segment that contains the Arg side chains and describe the properties of the amino acids in the sequence. Do this by scrolling along the sequence window in iCn3D (shown below) until you find the labeled Arg shown in the model.

- Answer
-
.
Is the helix amphiphilic? That is, are the Arg side chains all on one face of the helix and the nonpolar on the other? To find out, copy and paste the sequence of S4 (above) in this helical wheel predictor and run the program.
- Answer
-
.
Make a simplified view of the iCn3D model by hiding Domains 1-III to more readily see the contributions of S5 and S6 of Domain IV to the pore.
- open iCn3D and load 7K18.
- With your mouse or trackpad, choose Sequence and Annotation in the top menu bar
- Choose the Details tab
- Ctrl-Click the two sequences highlighted in yellow below for Domain IV and the toxin.
- Choose View from the top menu bar and then View Selection
- Choose Style, Background, Transparent


Solution
.
Noncovalent Interactions of LqhIII and Domain IV/VS
Now let's look at the actual interaction of the toxin with the Domain IV/VS of the channel. A closeup showing the interaction site is shown in Figures 3 b and c from the paper below. Panel C next to it shows the NMR-solution structures of the toxin in the absence of the channel. Each structure determined is represented by a single color line color codes red at the C-terminus to blue at the N-terminus.
Panel b: CryoEM structure of the rNaV1.5C Domain IV/VS and LqhIII complex; Panel c: NMR structure of free LqhIII
Using this iCn3D model, describe the secondary structure of the bound toxin. How many pairs of cysteine residues are in the LqhIII toxin. Identify which cysteines are involved in the disulfide bonds. What effect do the disulfide bonds have on the beta sheet structure?
- Answer
-
.
What sections of the toxin in panel B make the closest interactions with the Domain IV/VS of the channel? Describe their conformation flexibility in the free toxin.
- Answer
-
.
The figure below shows an interactive iCn3D model of a surface rending of Domain IV of the rat sodium channel NaV1.5 bound to the LqhIII toxin (7k18).
Surface rending of Domain IV of the rat sodium channel NaV1.5 bound to the LqhIII toxin (7k18). (Copyright; author via source). Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...x5QRPEctT5zsW7.
The molecular surface and underlying secondary structure of the LqhIII toxin are shown in magenta, with key residues H15, H43, and K64 shown as CPK-colored sticks and labeled. Domain IV/VS of the channel is shown in cyan, with key amino acid side chains T1608, D1612, and Q1615 shown as colored and labeled sticks.
Comment on the shape and possible side chain interactions that contribute to high-affinity binding of the inhibitor to Domain IV/VS.
- Answer
-
.
Figure 3d from the paper below shows the detailed interactions between LqhIII and DIV-VS. Key residues shown in sticks were labeled. Interaction surfaces of the DIV-VS (blue) and the LqhIII (purple). Key residues for the interaction are shown in yellow shading and embedded stick
The figure below shows an interactive iCn3D model of the rat sodium channel NaV1.5 Domain IV bound to the LqhIII toxin (7k18).
Rat cardiac sodium channel NaV1.5C Domain IV/LqhIII toxin complex (7k18). (Copyright; author via source). Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/icn3d/share.html?JAjUQAZDZoQ5wiVX9
The toxin is shown in magenta. The segments are colored as follows: S1 is red, S2 is orange, S3 is yellow, S4 is cyan, S5 is brown and S6 is violet. Key amino acid pairs involved in the binding of the toxin to Domain IV are shown in sticks and labeled.
In summary, name and locate the amino acid residues that serve the following roles in the LhqIII toxin: DIV-VS interactions.
- Which amino acids in the toxin interact with D1612 (the paper describes the interaction as pincers surrounding D1612).
- The conserved negatively charged residue in the Nav1.5 channel
- What position is Thr in and what is thought to be its role in the mechanism?
- Answer
-
.
Comparison of Activated DomIV-Voltage sensor (VS) with Toxin-bound Partially activated DomIV-VS
Now we'll try to understand Figure 4, Conformational Change of DIV-VS, from the paper and pay special attention to the section of the text, “An intermediate-activated state of DIV-VS trapped by LqhIII” - Let’s dissect Figure 4 A and B.
Figure 4a/4b from the paper below shows the conformational change of Domain IV-Voltage sensor (DIV-VS) comparing the activated and partially activated state with the bound toxin.
Panel a shows the activated domain IV with key Arg side chains in S4. Panel B shows the partially activated domain IV with the same key Arg side chains in S4. The bound LqhIII is shown as a purple chain. In panels a and b, the:
-
activated Nav1.5 DIV-VS (the voltage sensing domain) is in grey (fig 4a)
-
the intermediate-activated Nav1.5DIV-VS is in blue (Fig 4b)
-
side chains of gating charges of Arg are shown in grey and blue sticks in 4a and in shades of blue sticks in 4b. Side chains in the ENC are shown in red, in the HCS in yellow, and in the INC in red;
-
the shift of each gating charge was indicated by black dashed lines between the structures in panels a and b.
The shift from the cytoplasmic to extracellular parts of the channel is shown in the region between the two panels. The black Rs in the activated DIV-VS(panel A) are further up in the diagram (towards the extracellular region) and further down in the partially activated DIV-VS bound to the toxin.
Locate the 6 arginines? (R1-R6) with the blue indicating the N atoms in the positively charged Arg side chain of S4 in Domain IV in one of the iCn3D models above. What do the following abbreviations mean? ENC, HCS, and INC.
- Answer
-
.
From Figure 4a to 4b, explain from an electrostatic viewpoint how the movement of the Args towards the extracellular region would promote the movement of Na+ ions inward. Explain how the movement of Na+ ions would be diminished in the presence of the toxin.
- Answer
-
.
Comparison of Active and Intermediate-Activated, and Intermediate/Resting state
Now consider Figures 4 C and D from the paper below:
4c: Superposition of NaV1.5 DIV-VS between the fully activated state and toxin-bound intermediate-activated state. Red arrows indicate the conformational changes.
4d: Superposition of the intermediate-activated NaV1.5 DIV-VS and resting-state NaVAb-VS
a. Locate the region in Figure c above that shifts the most from the fully activated to intermediate-activated state of the DIV-VS.
b. Describe the difference shown in Figure d between the intermediate-activated DIV-VS structure (blue) upon the resting state NaVAb-VS structure (orange)
c. What do these differences imply about the conformational states of the apo and toxin-bound channel?
- Answer
-
.
Summary
Why might the mode of action be specific for cardiac muscle cells as compared to other toxins that act on sodium channels in skeletal and nerve cells?
- Answer
-
.
After this guided research literature module, you can hopefully better understand the findings in the paper which are summarized in this abstract:
"Voltage-gated sodium (NaV) channels initiate action potentials in excitable cells, and their function is altered by potent gating-modifier toxins. The α-toxin LqhIII from the deathstalker scorpion inhibits fast inactivation of cardiac NaV1.5 channels with IC50 = 11.4 nM. Here we reveal the structure of LqhIII bound to NaV1.5 at 3.3 Å resolution by cryo-EM. LqhIII anchors on top of voltage-sensing domain IV, wedged between the S1-S2 and S3-S4 linkers, which traps the gating charges of the S4 segment in a unique intermediate-activated state stabilized by four ion-pairs. This conformational change is propagated inward to weaken binding of the fast inactivation gate and favor opening the activation gate. However, these changes do not permit Na+ permeation, revealing why LqhIII slows inactivation of NaV channels but does not open them. Our results provide important insights into the structural basis for gating-modifier toxin binding, voltage-sensor trapping, and fast inactivation of NaV channels."
Extensions
1. Interesting sidebar: https://www.sciencedirect.com/science/article/pii/S0021925819308300?via%3Dihub
Chlorotoxin (Cltx) is a 36-amino acid peptide that was originally isolated from Leiurus quinquestriatus venom (14) and has been shown to inhibit small conductance Cl− channels in colonic epithelial cells (14, 15). Cltx also inhibits Cl− fluxes across glioma membranes (13, 16). Immunohistochemical studies show that Cltx specifically and selectively binds to glioma cells (17) and radiolabeled Cltx targets tumor cells in mice bearing xenografted glioma tumors. Glioma cell migration and invasion into fetal brain aggregates is significantly reduced by Cltx (13). A recent survey of over 200 tissue biopsies from patients with various malignancies suggests that Cltx binds to the surface of gliomas and other embryologically related tumors of neuroectodermal origin (18) but not to normal brain.
2. Deathstalker scorpion venom also contains chlorotoxin - This is a very interesting story.
Note and remember LqhIII is an alpha toxin
3. https://www.sciencedaily.com/releases/2010/08/100811125947.htm
4. How much is deathstalker venom worth?
- 94$ for 5 ug agitoxin; https://www.scbt.com/p/agitoxin-78207-24-6
- https://news.stanford.edu/2019/06/10/healing-compounds-scorpion-venom/ : “For the past 45 years, Possani has focused on identifying compounds with pharmacological potential in scorpion venom. His group has previously uncovered potent antibiotics, insecticides and anti-malarial agents hidden in the arachnid’s poison.”
- https://www.timesnownews.com/viral/reason-why-scorpion-venom-is-one-of-the-most-expensive-liquids-in-the-world-will-surprise-you-article-99409440 : $39 million dollars a gallon
- The venom of the deathstalker scorpion, one of the most dangerous scorpions on the planet, costs $39 million dollars a gallon, making it the most expensive liquid on Earth.Apr 11, 2023
5. Looks like a great review article: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7277529/
6. Scorpion Venom: Detriments and Benefits
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7277529/pdf/biomedicines-08-00118.pdf
- https://hal.science/hal-03616273/document
- https://www.rcsb.org/structure/1bmr
- https://www.researchgate.net/publication/308013755_Scorpion-Toxins_Lqh_III
7. Lookfor possible therapeutic potential here https://www.venomdoc.com/
8. Very cool venom graphic - https://www.venomdoc.com/new-page-2
9. The Toxicogenomic Multiverse: Convergent Recruitment of Proteins Into Animal Venoms: https://static1.squarespace.com/static/55a239e2e4b0b3a7ae106f25/t/59814ceae6f2e10bc7ada5f1/1501646072821/2009_Fry_Toxicogenomic_multiverse.pdf
10. The deathstalker scorpion venom alone has been found to have several different kinds of toxins including chlorotoxin (inhibit chloride channels), charybdotoxin (inhibit potassium channels), and agitoxins (affect sodium channels).. https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/leiurus-quinquestriatus. Chlorotoxin was found to selectively bind to glioma cells and serve as a marker for glioblastoma. https://www.acs.org/molecule-of-the-week/archive/c/chlorotoxin.html#:~:text=Strichartz%20at%20Harvard%20Medical%20School,diagnosing%20and%20treating%20some%20cancers. This feature of the scorpion venom was developed by J.M. Olson at Fred Hutchinson Cancer Center (Seattle) as a product called Tumor Paint https://www.fredhutch.org/en/news/center-news/2014/09/tumor-paint-US-trial.html


.png?revision=1)
.png?revision=1&size=bestfit&width=445&height=269)
.png?revision=1&size=bestfit&width=322&height=273)