19: Chemical Control of Microbial Growth
- Page ID
- 107329
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Learn about disinfectants and the various factors that need to be considered when choosing a disinfectant.
- Set up an experiment to determine the effectiveness of five different common disinfectants.
- Learn how to determine antibiotic susceptibility using the Kirby-Bauer method.
- Set up an experiment to test for antibiotic production by three different strains of Streptomyces.
Introduction
The use of chemicals to control microbial growth dates back at least as far as the 1800’s. Tincture of iodine was used as antiseptic during the Civil War, and Joseph Lister established the practice of aseptic surgery using a disinfectant known as carbolic acid (phenol) in the 1860’s. Since that time, many types of disinfectants (agents that are used to eliminate or kill vegetative cells on surfaces) and antiseptics (agents that are used to eliminate or reduce vegetative cells on living tissue) have been used. Although disinfectants and antiseptics may be effective at killing vegetative cells, they do not usually achieve sterilization.
Various factors need to be considered when choosing a disinfectant or antiseptic. It is very important to know which microbes are present to determine what type of disinfectant would work best. It is also important to realize that the effectiveness of a particular disinfectant may be affected by pH, temperature, concentration, and exposure time. Ideal disinfectants should be effective against the particular contaminants present, usable at a low concentration, require a relatively short exposure time, and have a long shelf life. It should also be water soluble, non-toxic to humans and animals, and cost-effective.
The efficacy of a disinfectant or antiseptic can be tested in several ways. One way is to inoculate an agar plate with a lawn of bacteria and add filter paper disks that have been moistened with the disinfectant being tested. This is known as the filter paper disk method, or agar disk diffusion assay. After incubation, plates are observed for the presence of a zone of inhibition (area around a disk where no microbial growth is detected). Generally speaking, the larger the zone, the more effective the disinfectant is against that particular microbe. However, other factors such as the solubility of the test agent and the molecular weight of the disinfectant molecules (which determines the diffusion rate of the disinfectant through the agar) can also affect results.
.png?revision=1&size=bestfit&width=701&height=304)
The use of antimicrobial agents to treat infections began in the early 1900’s, when Paul Ehrlich developed Salvarsan to treat individuals infected with Treponema pallidum, the spirochete that causes syphilis. In 1928, Alexander Fleming later observed that the Penicillium mold growing on his agar plates could inhibit the growth of bacteria: years later penicillin was purified and used to treat many types of infections. Since this time, many other antimicrobial agents have been used to treat a wide variety of bacterial infections. Antibiotic producers include many types of fungi (Penicillium, Cephalosporium) and bacteria (Bacillus, Streptomyces). In addition, many antimicrobial agents currently used to treat infections are either synthetic (made in a laboratory) or semisynthetic (a modification of a naturally-produced antibiotic). Today there are over 100 different antimicrobials that are used to treat infectious diseases. These include broad-spectrum and narrow-spectrum antimicrobial drugs (see chart below). Narrow-spectrum drugs are more desirable to use whenever possible because they target the pathogen more specifically and do less damage to the normal microbiota; broad-spectrum drugs are used when the cause of the infection is unknown or when other antibiotics are not effective.
Activity spectra of the major classes of antibiotics
| Mycobacteria | Gram Negative | Gram Positive | Chlamydiae | Rickettsiae | |
| Tetracyclines | ✓ | ✓ | ✓ | ✓ | |
| Streptomycin | ✓ | ✓ | |||
| Penicillin | ✓ | ✓ | ✓ | ||
| Sulfonamides, Quinolones, Cephalosporins | ✓ | ✓ | |||
| Vancomycin | ✓ | ||||
| Polymyxin | ✓ | ||||
| Isoniazid | ✓ |
It is important to remember that not all antibiotics are effective at killing all types of bacteria. Bacteria may have intrinsic resistance to a particular antibiotic. For example, gram negative bacteria are intrinsically resistant to vancomycin because the drug cannot penetrate the outer membrane of the gram negative cell wall. Also, the misuse and overuse of antibiotics has led to the evolution of resistance among bacteria by selecting for individual cells within a population that are not affected by the drug. This acquired resistance can occur in several ways, including through transformation, conjugation and mutation. Antibiotic-resistant bacteria have become a major problem of growing concern in health care, as it is often difficult (or impossible) to treat bacterial infections caused by these microbes (for example, multidrug-resistant Staphylococcus aureus, or MRSA). Therefore clinical isolates are often tested for their antibiotic susceptibility in a laboratory setting so that health care providers can choose an appropriate drug to treat a particular infection.
There are several ways to determine antibiotic susceptibility in a laboratory setting—one common test is called the Kirby-Bauer method. This method is similar to the filter paper disk method used to test disinfectants, except that it uses filter paper disks impregnated with a known concentration of an antimicrobial compound. It also uses Mueller-Hinton agar, and is often performed with larger (150 mm) petri dishes that allow for the testing of several antibiotics simultaneously. When performing the Kirby-Bauer method, it is important to measure the size of the zones of inhibition and compare them to a set of standardized values established by the Clinical Laboratory Standards Institute (CLSI).
Concentration-Dependent Drug Susceptibility
There are two methods that can be used to assess the most optimal concentration of antimicrobial drug to be used.
First, the E test uses a strip that is impregnated with decreasing concentrations of the antimicrobial drug. This test is performed to determine the minimum inhibitory concentration (MIC) or the lowest concentration of a drug required for prevention of microbial growth. A plate is prepares such that the E test strip is placed onto a solid medium that has been uniformly inoculated.
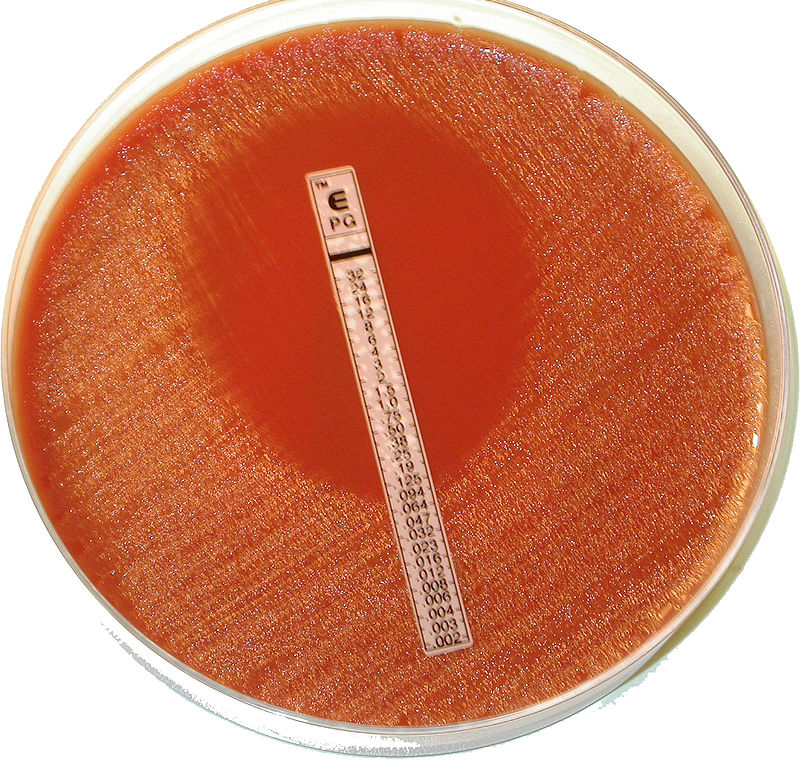
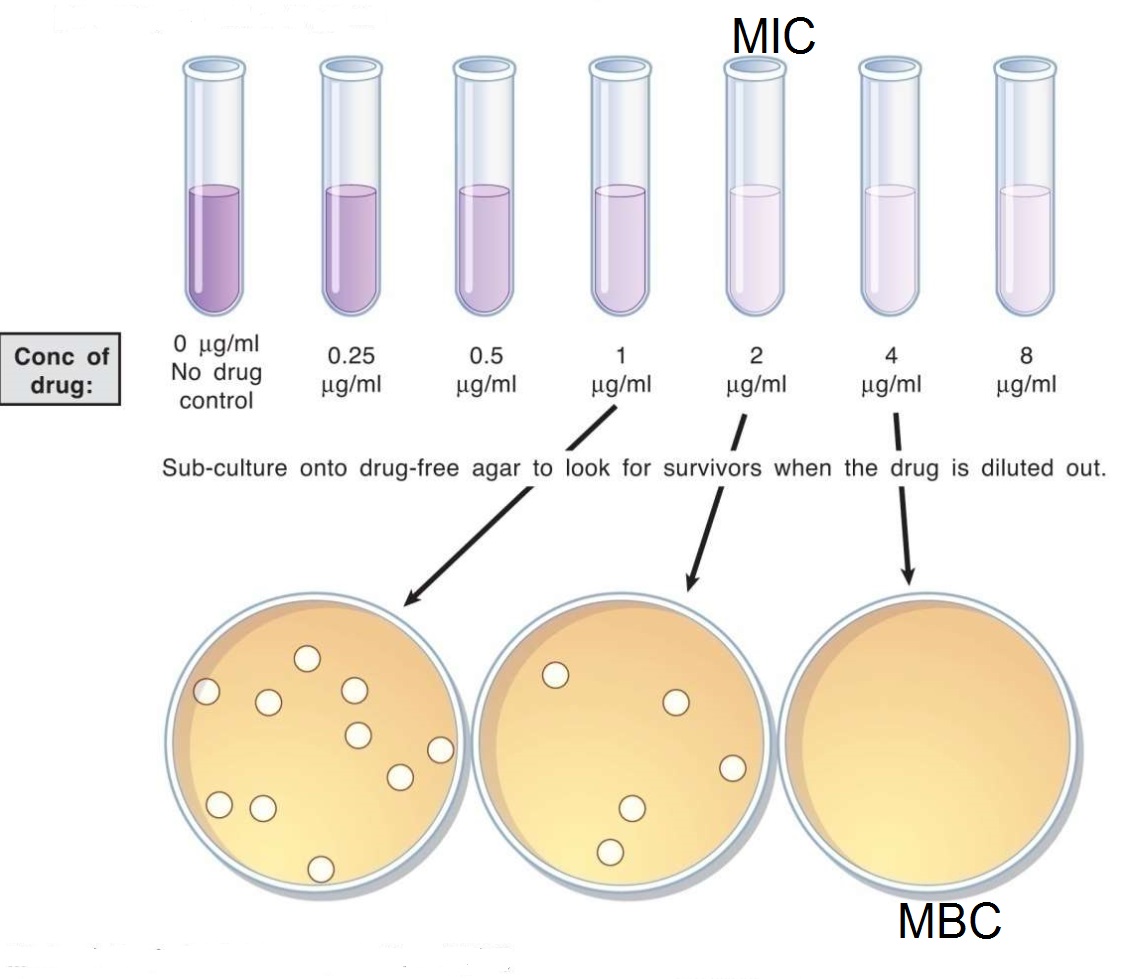
The second method for determining MIC using liquid media with decreasing concentrations of the antimicrobial drug. This is called the broth dilution test. The specimen is inoculated into various dilutions of antimicrobial drug in liquid media and assessed for the absence of growth. As a secondary measure, dilutions which do not appear to have growth are subcultured onto media without antimicrobial drugs. This measure serves two purposes: 1) Identifies the minimum inhibitory concentration of a drug, 2) Identifies whether the drug is bactericidal (kills microbes) or bacteriostatic (inhibits microbial growth).
Key Terms
disinfectant, antiseptic, filter paper disk method (agar disk diffusion assay), zone of inhibition, Kirby-Bauer method, broad-spectrum antimicrobial drugs, narrow-spectrum antimicrobial drugs, susceptibility, resistance, intrinsic resistance, acquired resistance, E test, minimum inhibitory concentration (MIC), broth dilution test, bactericidal, bacteriostatic
PROCEDURES
A. Disinfectants
For this exercise, you will perform a filter paper disk diffusion assay to determine the effectiveness of 3 different types of disinfectants.
Materials: 1 TSA plate/student, liquid cultures of Staphylococcus aureus and Escherichia coli, sterile cotton swabs, filter paper disks, 3 different disinfectants, sterile water, forceps, and beakers with 70% ethanol
Write down the names of the 3 disinfectants you will test in the chart below.
| Disinfectant # | Name | Concentration | Active ingredient/class of disinfectant |
| 4 | Sterile distilled water (control) |
- Choose one of the bacterial species listed above. Use a cotton swab to inoculate a lawn of bacteria on your TSA plate. Discard swabs in the beaker provided at your table.
- Label your TSA plate with the name of the microbe you are using, and numbers 1-4 evenly spread out on the plate (see demo plate done by your instructor). The numbers (and corresponding disks) should not be too close to the edges of the plate.
- Bring your inoculated plate up to the front table, where you will be adding filter paper disks soaked in disinfectants.
- Before each use, forceps should be flame sterilized as follows:
- Remove forceps from beaker of alcohol. Keep the tips angled down at all times
- Put the forceps in the Bunsen burner flame just to ignite the alcohol. NOTE: You are not heating the forceps, just igniting the alcohol.
- Keep a careful eye on the forceps (hold them steady in one location) until all the alcohol has burned off (this will be very quick). To avoid fires, do not hold them over any beakers of alcohol!
- Once the alcohol has burned off, use the forceps to pick up one filter paper disk from the glass Petri dish.
- Dip the disk into disinfectant #1.
- NOTE: Just touch the surface of the liquid—the disinfectant will soak into the disk by capillary action. It is important to not have the disks too wet when you place them on your TSA plate.
- Place disinfectant #1 on the appropriate area of the agar plate. Tap it down gently with the forceps to ensure that it adheres to the surface of the agar.
- Repeat this step for all disinfectants.
- NOTE: If you want to test some other product, just omit one of the disinfectants in the front of the room.
- Add a filter paper disk that has been dipped in sterile water to the area of the plate labeled “#4”- this will serve as your negative control.
- After each use, place the forceps back into the beaker with 70% ethanol. DO NOT heat them after use before returning them to the beakers.
- Incubate plates (inverted, as usual) until the next lab period.
-
B. Antibiotic Susceptibility Testing
Materials: 1 Mueller-Hinton agar plate, liquid cultures of Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa, sterile cotton swabs, antibiotic disk dispensers.
- Work as a table for this exercise. Use a sterile cotton swab to inoculate the Mueller-Hinton agar plates with a lawn of bacteria for each species listed (one species/plate). Be sure to cover the entire area of the plate. This can best be accomplished by swabbing the entire surface of the plate three times, rotating the plate approximately 60º each time, and finishing the swabbing by going around the entire outer surface of the plate.
- After all plates are inoculated, your instructor will show you how to use the disk dispenser to introduce antibiotic disks onto the plates. Be careful to always keep the dispensers in the upright position.
- You will notice that each disk that is introduced to the plate is stamped with a letter and a number—these indicate the type of antibiotic as well as the concentration. For example, P10 is an abbreviation for 10 units of penicillin; PIP-100 is an abbreviation for 100 mcg (micrograms) of piperacillin.
- Return to your lab table, and use your inoculation loop or needle to gently push down on the disks to ensure that they are adhered to the agar surface.
- NOTE: Flame your loop in between plates to avoid cross-contamination
- Incubate the Mueller-Hinton agar plates (inverted) until the next lab period.
C. Determining the MIC for an Antimicrobial Drug
1. Starting with the highest concentration of drug, perform 2-fold serial dilutions.
a) Remove 5mL of media containing the antimicrobial drug and add it to 5mL of media without drug.
b) Mix by pipeting up and down, transfer 5mL of the diluted drug-containing media to 5mL of media without drug.
c) Repeat this process until the desired dilution is achieved.
d) Choose one of the sources of inoculum and inoculate all tubes of media.
e) Incubate media for 24-36 hours.
f) After incubation, streak out any cultures that appear to be absent of growth on drug-free media to determine if there are any microbes present and whether the drug is bactericidal or bacteriostatic. Incubate plates for 24-36 hours, inverted.
Dilution Nomenclature
The first tube of media we use is undiluted drug. For example, let's say we are starting with 16ug/mL of drug A. When we perform 2-fold serial dilutions, the first dilution is called a 1-to-2 dilution or 1:2 dilution. Since we are effectively cutting the concentration in half, the resulting concentration of drug A is 8ug/mL. When you perform the next dilution, you take 5mL of your 1:2 solution and transfer it to an equal volume of media without drug. This dilution is called a 1:4 dilution. The next dilution would be 1:8, 1:16, 1:32, and so on. This type of dilution is called a 'two-fold dilution' because the concentration of the drug is reduced by two-fold with each dilution. Correspondingly, the concentrations of drug A at 1:4, 1:8, 1:16, and 1:32 are 4ug/mL, 2ug/mL, 1ug/mL, and 0.5ug/mL, respectively. Note that the higher the dilution, the LESS drug is in solution.
A. Disinfectant experiment
Use the spaces below to record your observations of the appearance of your own TSA plate.
| Disk # | Name | Observations |
| 1 | ||
| 2 | ||
| 3 | ||
| 4 |
Based on your results, which disinfectant(s) worked best against the bacteria you tested?
Which disinfectant(s) were least effective?
Compare your plate with that of your lab partner. Did you see the same pattern of zones of inhibition for both bacteria tested? Explain.
B. Antibiotic susceptibility testing
Observe the Mueller-Hinton plates, and note any differences in their overall appearance. Which plate seems to have the most zones of inhibition?
Which has the least?
Do you see any small colonies within the zones of inhibition on any of your plates? What do these colonies represent?
For each plate, measure zones of inhibition for 3-4 antibiotics as follows:
1. With a metric ruler, measure the diameter of the zone of inhibition. Express the values in millimeters (mm). It is best to choose some small, medium-sized, and large zones for comparison.
2. Use the charts provided by your instructor to look up the interpretation for each of the zones you measured. Record measurements and interpretations in the chart below.
.png?revision=1&size=bestfit&width=500&height=263)
Figure 8.3.1: Measuring zones of inhibition
| Bacterial species | Disk label | Antibiotic/concentration | Zone of inhibition (mm) | Interpretation (R, I, S) |
| E. coli | ||||
| S. aureus | ||||
| P. aeruginosa | ||||
Based on your results, do all antibiotics work equally as well against all types of bacteria? Explain.
Why do we need to look up values in the charts for each antibiotic?
C. Determining the MIC for an Antimicrobial Drug
Examine the liquid media and plates for evidence of antibiotic activity. Report the minimum inhibitory concentration of the tested drug on the microbe you chose to test.
LAB ASSIGNMENT
1. True or False: All antibiotics will be equally effective against Gram positive and Gram negative bacteria.
2. Do you think that a disinfectant or antiseptic that works well on an agar plate always works well in a real world setting? Why or why not?
3. You are asked by your place of employment to order a disinfectant that will be used for daily cleaning. What are some of the factors that you will consider when choosing which one to order?
4. Give an example of when a health-care provider might choose a broad-spectrum antibiotic over a narrow spectrum antibiotic.
5. What are some of the ways that antibiotics are misused or overused?
6. How would treatment with tetracycline affect penicillin therapy?
7. The following results were obtained from a disk-diffusion test using novel antibiotics against a bacterium isolated from human sputum. Which drug should be used to treat this bacterial infection. Briefly explain.
Antibiotic Zone of Inhibition in mm
- Cheetomaniazillin: 6
- Pizzapropham: 18
- Chocolazapam: 11
- Tacomacillin: 18
Attributions
This chapter is shared under a CC BY-NC-SA license and was authored, remixed, and/or curated by LibreTexts.
- 8.1: Introduction by Joan Petersen & Susan McLaughlin is licensed CC BY-NC-SA 4.0.
- 8.2: Procedures by Joan Petersen & Susan McLaughlin is licensed CC BY-NC-SA 4.0
- 8.3: Results by Joan Petersen & Susan McLaughlin is licensed CC BY-NC-SA 4.0.
- 8.4: Review Questions by Joan Petersen & Susan McLaughlin is licensed CC BY-NC-SA 4.0.

